Cryopreservation Case Report: Arlene Frances Fried

This is an archived case report. It does not reflect Alcor’s current best practices for cryopreservation nor current standards for case reporting. It remains accessible for historical purposes, but, to find out more about Alcor’s current procedures, see What is Cryonics.
This technical case report, first published here, is a uniquely comprehensive report of an actual cryonics case performed under nearly ideal conditions given the technology of its era. The report concludes that viability of the brain by contemporary medical criteria was likely maintained throughout the duration of the transport phase of the procedure. See also the personal account of this case by Arlene Fried’s daughter: Her Blue Eyes Will Sparkle.
Alcor #: A-1049
Patient Name: Arlene Frances Fried
Date of Birth: 03/30/22
Date of Cryopreservation: 06/09/1990
Report prepared by: Michael Darwin, January 2006
CONTENTS:
- Background History and Informed Consent
- Medical History
- Dehydration and Agonal Course
- Cardiopulmonary Arrest and Pronouncement of Legal Death
- Transport
- Total Body Washout Perfusate Preparation
- Total Body Washout (TBW)
- Air Transport
- Gross Assessment on Arrival
- Cryoprotective Perfusate Preparation
- Surgical Procedures
- Cooling to Dry Ice Temperature
- Cooling to -196°C
- Laboratory Evaluations
- Discussion
- Summary
- References
- Addendum: Laboratory Data
Background History and Informed Consent
Mrs. Fried first became aware of cryonics as a result of the involvement of her daughter Linda Chamberlain who, with her husband Fred Chamberlain, founded the Alcor Life Extension Foundation in 1972 (all dates are CE, all clock times 24-hour). At her daughter’s and son-in-law’s urging Mrs. Fried began making cryopreservation arrangements in April of 1983. These arrangements were completed and Mrs. Fried was approved for suspension membership by Alcor on 12 January, 1986. Mrs. Fried stated at that time that her primary purpose in making suspension arrangements was to please her daughter and son-in-law.
During the interval from the time she first began making suspension arrangements, until the diagnosis of her final illness in January of 1990, Mrs. Fried had numerous contacts with Alcor personnel and attended several Alcor functions. She also regularly read the Alcor newsletter Cryonics magazine. When Mrs. Fried was diagnosed with adenocarcinoma of the lung she became more interested in cryonics and stated that, contrary to her previous position of having made arrangements with Alcor “to please her family” she now felt a genuine desire for cryopreservation. During this period she asked many questions about cryonics in general and Alcor’s procedures in particular and stated repeatedly that she felt that cryonics offered her real hope. She also took a very active role in arranging her terminal care in ways that would increase her chances of a quality cryopreservation.
Further evidence of the change in Mrs. Fried’s attitude vis-à-vis her cryopreservation arrangements was her unsolicited and spontaneous request that the non-cryopreserved portion of her body be used in any way that might advance the state of the art of either human cryopreservation for future medical rescue, or organ cryopreservation for transplantation. To this end, a protocol was established wherein one of the patient’s kidneys would be air-shipped to a qualified cooperating organ cryopreservation research laboratory following completion of cryoprotective perfusion for viability assessment using the Na+/K+ ratio [1] (to evaluate the effectiveness of Alcor transport procedures) as well as to serve as a source of human renal tissue for evaluation of the toxicity of cryoprotectant mixtures being employed in experimental attempts to vitrify mammalian kidneys for long-term organ cryopreservation.
Mrs. Fried’s long acquaintance with both cryonics and Alcor, as well as her aggressive involvement in her own care, particularly as it related to her impending cryopreservation, establish her informed consent.
Mrs. Fried is a 68-year-old female who enjoyed good health until early December of 1989 at which time she developed a flu-like illness while on a vacation cruise in the Atlantic. The illness consisted of bronchitis-like symptoms; slight malaise without fever, slight hemoptysis, and occasional yellow sputum. While shipboard she stated that she received a course of (sic) “Penicillin antibiotic” with minimal improvement. She was feeling slightly improved until the evening of 1-11-90 at which time she experienced numbness of her right nare. On the morning of 1-12-90 she experienced Jacksonian seizures involving the right side of her face and right upper arm. These seizures were followed by what the patient described as “severe tremors” affecting the right side of her face and arms accompanied shortly thereafter by transient Broca’s aphasia. She was transported by paramedic ambulance to the Emergency Department (ED) of Sonoma Valley Hospital where she experienced additional focal seizures of the right arm and face without loss of consciousness. She was medicated with intravenous (IV) diazepam which was effective in halting the seizures.
Subsequently during her 1-12-90 admission the patient was diagnosed with metastatic adenocarcinoma of the upper lobe of the left lung. There was also evidence suggestive of early chronic obstructive pulmonary disease (COPD). The patient was informed she was terminally ill and decided, in consultation with her oncologist Dr. Mark Bosdick, M.D, to forego what she was advised would likely be futile and quality of life eroding attempts at curative or possibly life-extending chemotherapy and radiotherapy. She was enrolled in a hospice program with Valley of the Moon Hospice shortly after her diagnosis was established.
Computerized Tomogram (CT) scan during this admission revealed a ring enhancing lesion of the right parietal lobe white matter consistent with metastatic disease. A bone scan and spot X-ray films revealed evidence of metastatic disease at T-3 thru T-5 and at T-11.
The patient was discharged to home 1 January, 1990 on 300 mg phenytoin daily and dexamethasone 4 mg b.i.d.
The patient’s past medical history is remarkable for the following:
• 1.5 packs of cigarettes per day for 50 years (75 pack years)
• Hypertriglyceridemia and hypercholesterolemia with asymptomatic arteriosclerotic cardiovascular disease
• Bladder suspension in April, 1985
• Pyloric channel ulcer diagnosed in December, 1983
• Hysterectomy with ovarioectomy in 1965
• Ovarian cystectomy with ovarioectomy in 1947
• Tonsillectomy and adenoidectomy in 1928
On 25 January, 1990 the patient was re-admitted with progressive swelling and discomfort of the right upper arm as well as absent radial, brachial and axillary pulses. Subsequent ultrasound evaluation disclosed deep vein thrombi of the veins of the left upper arm extending to the internal jugular vein with accompanying marked stenosis of the subclavian artery on the left side, most probably at its origin. The patient was treated with IV heparin infusion with good results (resolution of edema and restoration of axillary, brachial and radial arterial pulses). The patient was discharged home on 2 February, 1990 with 7.5 mg per day of Coumadin added her to regimen to control what was thought to be a coagulopathy secondary to her adenocarcinoma.
On 6 February 1990 the patient was admitted with severe, intractable mid-back pain apparently secondary to her vertebral metastatic disease. At this time a decision was made to begin a course of palliative radiotherapy to the spine between T-3 and T-5. A course of whole-brain radiation (4,000 rads) and palliative chemotherapy with cisplatin was also scheduled. During this hospitalization a Port A Cath® portal central venous catheter was implanted (catheter tip positioned in the right superior vena cava) to facilitate venous access for chemotherapy and for laboratory studies (i.e., frequent CBC, serum chemistries, PTTs and phenytonin levels).
Another hospitalization followed on 10 March, 1990 for severe, intractable nausea and vomiting with accompanying dehydration. Management of the patient’s nausea proved difficult and she was not discharged until 19 March, 1990 at which time her nausea had resolved and it was noted that her response to radiotherapy had been good with no further occurrence of pain in her thoracic spine. Discharge medications were: Dilantin (Parke-Davis brand phenytoin), 100 mg t.i.d., metoclopramide, 10 mg. q.i.d., a.c. and h.s., RoxanolTM morphine sulphate elixir) 30 mg q. 8 hours p.r.n. for pain, ranitidine 150 mg at h.s., Compazine (prochlorperazine edisylate) q. 4 hours p.r.n. for nausea, Decadron (dexamethasone) 4 mg b.i.d., and Coumadin (warfarin) 7.5 mg every other day to be monitored with weekly prothrombin times (PTTs). The patient’s final hospitalization occurred on 21 March, 1990 as a result of multiple focal motor seizures which progressed to status epilepticus. She was discharged on 24 March, 1990 with her Dilantin dosage increased to 200 mg b.i.d.
Following her discharge on 24 March the patient’s condition improved to the point that she was briefly ambulatory and her nutritional status improved. However, throughout May the patient’s condition steadily worsened with weakness, anorexia and cachexia largely confining the patient to a reclining chair in her kitchen.
It is significant to note that the patient experienced the complete loss of all hair on her head as a result of chemotherapy induced alopecia. She did not re-grow any meaningful amount of hair during the remainder of her illness. This is significant because the absence of any hair on the head might reasonably be expected to materially improve heat exchange during external cooling at the start of post-arrest cardiopulmonary support and transport.
At the end of May the patient’s quality of life had reached a point that she stated was no longer acceptable and she began to discuss suicide via overdose with RoxanolTM and/or carbon monoxide. The impact of this course of action on her cryonics arrangements (i.e., making her a coroner’s case and likely subject to autopsy) was discussed and the patient reached a decision to end her life via dehydration by foregoing significant intake of food and fluids. This decision was implemented by the patient beginning on 30 May, 1990.
Following this decision the patient’s intake and output (I/O) and vital signs were charted daily by family up until the last four days of her dehydration at which time vitals were evaluated more frequently. Vitals were taken hourly during the last 6 hours of life. Fluid intake was typically in the range of 200 cc to 300 cc per day until the last three days of life when it dropped to 20 cc to 30 cc per day. Urine output varied between 200 cc and 50 cc per day with the patient voiding 250 cc of extremely concentrated molasses-colored urine on 9 June, 1990, the day of her cardiac arrest.
Throughout the period of the patient’s self-imposed dehydration the patient was repeatedly reassured that this was not a course of action she should feel compelled to continue and she was reassured that she could resume hydration at any time without any adverse impact on her cryopreservation arrangements. The patient repeatedly declined these offers to resume eating and drinking stating that her quality of life was unacceptable.
It is worth noting here that the patient developed several methods of coping with extreme thirst without resorting to increased doses of narcotic analgesics, or the use of anxieolytics or other sedating drugs. She stated that her reason for avoiding sedation was because she wished to (sic) “remain alert and mentally unimpaired until the end in order to spend as much of her remaining time possible with her family and friends.” These coping strategies consisted of rinsing her mouth with a favorite beverage (usually coffee) and sucking on ice chips; often spitting the resulting water into an emesis basin.
The patient remained conscious and appropriately responsive until the last 24 hours of life with the exception of a short period of agitation, delirium and confusion (~10 minutes duration) which occurred upon waking from a nap on 03 June. The patient recovered from this episode with verbal reassurance from her daughter and son-in-law, and repeated statements from them helping her to become oriented as to persons present, as well as to time and place. The patient’s recovery from this episode of altered sensorium was remarkable and she continued to frequently reminisce about her life and converse at a high level of function until the last 48 hours of life. Forty-eight hours before cardiac arrest the patient began to sleep most of the day and became increasingly difficult to rouse but remained oriented x 3.
Due to concerns on the part of the family that the patient was “anuric” because she had not voided for 3 days and the transient alterations in her sensorium the family requested that a Standby Team be deployed on 02 June. Because it was impossible to assess the patient well remotely and because the hospice nurse was unavailable to give a clinical assessment of the patient’s likely agonal course and time to cardiac arrest, a decision was made to deploy the Standby Team Leader and one other skilled team member and this was done on 03 June.
Cardiopulmonary Arrest and Pronouncement of Legal Death
At 1600 on 9 June, the patient was noted to be obtunded (level of consciousness (LOC) = 3 on the Glasgow Coma Scale (GCS)) and diaphoretic, with vital signs as follows: B.P. 50/38, pulse 140, and respirations 16. Capillary refill time was 2-3 seconds and the extremities were dusky and mottled with marked pedal cyanosis. Pupils were mid-position and were sluggishly responsive to light. Vital signs up to the time of cardiopulmonary arrest are show in both tabular and graphic form below:

A-1049 Vitals

Graphic Agonal Vitals: This patient’s apical heart rate (HR), versus mean arterial pressure (MAP), are a textbook presentation of the course of hypovolemic shock, particularly in the setting of compromised coronary circulation. Note, that as MAP declines, HR increases to the patient’s maximum HR. Once MAP declines to ~50 mmHg, or below, coronary perfusion becomes inadequate, MAP begins to rapidly deteriorate, HR falls precipitously, and cardiac arrest occurs. In the young patient or the well-conditioned older patient peak HR will typically be higher, the tolerable period of MAP at or below 50 mmHg will be far longer, and the agonal course may be prolonged by a long period of bradycardia before cardiac arrest occurs. This may result in many hours of inadequate systemic and cerebral perfusion (shock) before legal death occurs. NOTE: Cuff pressures may not be accurate (or even obtainable) in many patients in profound agonal shock. Thus a MAP of <50 mmHg obtained via brachial sphygmomanometery may be 30 to 40 mmHg lower than true central (vital organ) MAP.

Photo 1: As the patient becomes frankly agonal, transport medications are prepared for administration. Preparation of these medications just prior to cardiopulmonary arrest saves a considerable amount of time and facilitates their administration at the start of reperfusion when cardiopulmonary support (CPS) is initiated.

Photo 2: The patient was a home hospice patient and experienced medico-legal death in her home. It was her desire that assets to provide for immediate post-arrest CPS, medication, and exterrnal cooling be deployed in her home. To this end, her living room was set up as the emergency response area several weeks prior to medico-legal death. The dining room table in the foreground was used to lay out airway management supplies and was used to organize and prepare transport medications for administration. The background shows the patient in the portable ice bath (PIB). Personnel not in scrubs were relatives and Alcor volunteers who helped with various supportive tasks such as monitoring oxygen supplies, assisting with icing, taking photographs, and scribing temperature readings at predeternmined intervals.
Cardiac arrest occurred at 1747 on 9 June, 1990 and legal death was pronounced by Terry Hauptman, R.N., the attending Registry nurse at that time. (1747 has been used as the arrest time wherever decimal hours post-arrest are calculated.)
Immediately following the pronouncement of legal death the patient was moved from the reclining chair in which she arrested and was quickly carried to the portable ice bath (PIB) in an adjacent room. A Brunswick esophageal gastric tube airway (EGTA) with an attached Fenem end-tidal CO2 (EtCO2) detector was quickly placed to facilitate ventilation and a model M/1005 Michigan Instruments Thumper extensively modified by the manufacturer to deliver High Impulse CPR was installed. Cardiopulmonary support was initiated at 1750 at a rate of 80 compressions per minute with a systole/diastole ratio of 30:70 (30% duty cycle). Chest deflection was initially set at “4” on the Thumper and then reset at 1800 to “5” (yielding approximately a 6 cm depth of compression). The force on chest control was set to 90 psi (~ 5.5 kg/cm2) yielding a “square” wave-form which has been shown in both animal and human clinical studies to improve cardiac output during CPR [2-5].Ventilation was via an integrated time-cycled (2 second inspiration) positive pressure ventilator at a rate of 16 breaths per minute and a minute volume of ~10 L/min via EGTA at a continuous inspiratory pressure of 30 cm of H2O and an FiO2 of 0.8. PEEP was not used.
There was an immediate return of agonal gasping upon the initiation of CPS accompanied by “pinking-up” of the patient with the skin on the thorax and abdomen. The face appearing ruddy and well perfused. The lips and buccal mucosa could not be observed due to the presence of the EGTA mask. This was in sharp contrast to the duskiness and cyanosis observed during the hours of ante mortem shock which preceded cardiac arrest.
Simultaneous with the start of CPS the patient was externally cooled with approximately 60 kg of water ice to which 5 gallons of room temperature water was added. An array of tubing driven by a Sears Model # 563-269500 submersible utility pump (36 GPM @ 10′ head) and terminating in sprinkler heads was used to circulate ice-cold water in the PIB over the patient, particularly over the head and anatomical areas with large, high flow vessels near the surface of the skin: the groin, neck, and axilla. This device is referred to as the Surface Convection Cooling Device (SCCD). Measured PIB water temperature is typically 2.5°C measured at the bottom of the bath near the head-end and 1.5°C at the output of the submersible pump [6].

Photo 3: The hospice nurse examines the ETCO2 detector (the disc-shaped plastic device under the nurse’s left thumb). Note the surface convection cooling device (SCCD) tubing with with two cold water discharge heads over each carotid artery. A similar line is under the patient with two discharge heads delivering 0-2° C water to the patient’s cranial vault.
Hi-impulse Thumper support terminated at 1802 due to blow-off of an internal gas line resulting in failure of the unit. Manual CPR was immediately begun per AHA Guidelines [7] and a conventional Michigan Instruments Life-Aid Model 1004 Thumper was installed at 1806. Model 1004 Thumper compression parameters were as follows: duty cycle 50%, sternal deflection 7 cm with the force on chest control set at 60 psi (~ 4.5 kg/cm2). Time cycled positive pressure ventilation (2 second inspirations) was continued at a rate of 16 breaths per minute, a Minute Volume of 10 L/min, an FiO2 of 0.8, and a continuous inspiratory pressure of 30 cm of H2O.
Initial response to CPS was judged to be excellent as indicated by prompt return of agonal gasping, pinking-up of the skin, and an EtCO2 of 2-3% (5% is optimum). A venous blood sample drawn at 1825 via the Port-A-Cath was bright red, indicating good ventilation and perfusion. Carotid and femoral pulses, as evaluated by palpation, were noted to be strong and in synchrony with the Thumper throughout CPS; the last assessment was done during vehicular transport to the mortuary for Total Body Washout (TBW).
IV access was via an implanted Port A Cath® using a 16 g. Sorenson thin-wall dialysis needle to puncture the Port A Cath® septum and facilitate rapid IV administration of transport medications.
Administration of transport medications began at 1754 with IV push medications as follows: sodium pentobarbital 1092 mg, 2 gm deferoxamine at 1755, 400 µg nimodipine at 1754, 4368 mg sodium citrate at 1755, 2912 mg Trolox at 1819, 4,550 mg ascorbic acid (Cevalin) at 18:12, 15,288 IU sodium heparin at 1755, 1 g methylprednisolone at 1756, 109 mg chloropromazine at 1755 and 2.55 mg metubine iodide at 1756. Continuous IV infusion of 500 cc of 0.3 M tromethamine (THAM) and 500 cc of 20% mannitol in water were begun at 1756 and 1758, respectively.
A venous blood sample drawn from the Port A Cath® at 1750 disclosed the following:

Evaluation of Baseline Blood Sample: A baseline blood sample drawn shortly after the start of CPS (but prior to the administration of any transport medications) is indicative of dehydration and emerging renal failure. The BUN and uric acid are markedly elevated at 47 mg/dl and 8.8 mg/dl, respectively, and the serum K+ is 9.7 mEq/l (a potentially lethal level). The serum creatinine is slightly elevated at 1.4 mg/dl (normal = 0.6 to 1.2 mg/dl). The serum total protein (TP), while appearing to be at the lower end of the normal reference range (6.0 to 8.0 g/dl) must be considered in the context of the patient’s profound dehydration and hemoconcentration as indicated by the serum osmolality of 358 mOsm/l. Normovolemic serum TP, consistent with this patient’s poor nutritional status, would be expected to be below 5.0 g/dl. Probable respiratory alkalosis is present with a pH of 7.65. Further discussion of these results is present in the section on Evaluation of Serum Chemistries near the end of this report.
At ~1838 SCCD augmented external cooling was temporarily discontinued due to lack of AC power during vehicular transport. At1840 the patient was loaded into a mortuary van which departed at 1845 to Duggan’s Mission Chapel Mortuary for femoral cutdown and TBW. Mechanical cardiopulmonary support and infusion of THAM and mannitol were continued en route to the mortuary and the infusions were completed at approximately 1857. Arrival at the mortuary occurred at 1853. Administration of large volume parenteral medications required pressure infusion due to elevated central venous pressure from CPS which precluded gravity infusion.

Photo 4: The patient was transported to the mortuary for total body washout (TBW) without interruption in closed chest mechanical CPS. In this photo personnel are preparing to lift the PIB into the back of the mortuary transport vehicle.

Photo 5: The patient, with CPS continuing, is loaded into the van provided for transport to the mortuary which was located approximately 5 minutes drive from the patient’s home.

Photo 6: The H-cylinder of oxygen driving the Thumper is loaded next to the PIB in the transport vehicle.

Photo 7: The patient in the PIB along with the oxygen bottle secured next to her is fully loaded into the vehicle and ready for transport to the mortuary for TBW.
The patient’s axillary temperature at the time of cardiac arrest was 38.3°C. The first manually recorded temperatures obtained during transport were logged at 1833 and were 24.0°C rectal and 24.8°C esophageal. Esophageal temperature was monitored with a Barnant Instrument Co. Digi-Sense (Cole-Parmer Cat. 8528-20) thermocouple thermometer employing a copper-constantan vinyl coated TC probe to measure esophageal temperature (Cole-Parmer Instrument Co. Model #8505-90). Rectal temperature was monitored with a prototype auto-logging thermometer. The patient’s temperature descent during external cooling is presented graphically below. Times shown are decimal hours post-arrest:
A-1049 Cooling Temperatures (Initial)

PIB-SCCD Facilitated Cooling: As can be seen from the graph above the patient’s rectal temperature declines evenly at a rate of 0.32°C/min during the first 20 minutes of CPS. Thereafter there is a ~3 minute rebound of rectal temperature to 35°C, followed by a slight plateau in the cooling rate. The rebound and plateau appear to correspond with failure of the High-impulse Thumper and substitution of manual CPS which occurred from 1802 to 1806; between 12 and 16 minutes into CPS and external cooling. It is possible that the anomaly in the rectal cooling curve observed 20 minutes into CPS is indicative of greatly reduced cardiac output as a result of the switch to manual CPS. The cooling rate declines sharply after 60 minutes of CPS to 0.13°C/min. This may be as a result of decreasing ∆T between the patient and the bath, a reduction in cardiac output due to a decay in the efficacy of CPS, or a combination of the two.
There is a sharp and expected increase in the rate of core cooling as measured by rectal temperature from and average of 0.025°C/min during the preceding 40 minutes of external CPS and external cooling to a rate of 1.0°C/min after the start of extracorporeal perfusion (TBW) and intravascular cooling.
SCCD supported external cooling was re-initiated after arrival at the mortuary at approximately 1906. The patient cooled at an average rate of 0.41°C/min during the first 12 minutes of external CPS and external cooling. The average rate of cooling was 0.33°C/min during the first 43 minutes of CPS and external cooling declining markedly to 0.13°C/min between 60 and 90 minutes into Transport.

Comparison Of Cooling Methods: Above are actual cooling curves for three adult human cryopreservation patients on Thumper support, using ice bags, the Portable Ice Bath (PIB), and the PIB augmented by SCCD (squid) cooling. Patient A-1133 weighed 56.8 kg, patient A-1169 weighed 57.3 kg, and patient A-1049 weighed 36.4 kg. As this data indicates PIB cooling is approximately two times as efficient as ice bag cooling. The SCCD appears to increase the rate of cooling by an additional 50%over that of the PIB (roughly adjusting for the difference in the patients’ body mass).

Photo 8: After arrival at the mortuary the PIB was placed atop the embalming table and preparations were made to carry out TBW. In the far right foreground the extracorporeal circuit is being assembled.
Thumper CPS was briefly interrupted between 1848 and 1849 when the H-oxygen cylinder supplying the unit became exhausted and was switched out for a fresh tank. Patient temperatures at the time of this interruption were 11.2 °C esophageal and 13.1 °C rectal.
At 2010 a small quantity (3cc-5 cc) of the blood-tinged foam characteristic of pulmonary edema was noted in the patient’s oropharynx. Pulmonary edema is known to develop rapidly (~10 minutes) during CPR with the severity increasing with the duration of CPR [8-11].
There was no evidence of erosion of the gastric mucosa, as evidenced by lack of gastric bleeding; aspiration of the gastric tube in the EGTA disclosed yellow cream colored gastric contents, presumably Maalox and gastric juices tinged with bile.
Total Body Washout Perfusate Preparation
The composition of the total body washout flush solution is given in Table I. Dry chemical perfusate components were prepared from reagent or medical grade chemicals weighed out using an Ohaus Centogram model 311, or an Ohaus Triple Beam 2610 g balance. Dry components were mixed with sterile water for injection USP, or sterile water for irrigation USP. Perfusates were sterilized by filtration into the oxygenator of the extracorporeal circuit through a Pall PP3802 0.20µ pre-bypass filter. A total of 20 liters of washout perfusate was prepared consisting of SHP-1 with Dextran-40 as the colloid. Perfusion with SHP-1 washout solution was followed with 6 liters of ViaSpan (Belzer UW) Cold Storage Solution.
TABLE 1
SHO-1 Total Body Washout Perfusate

Measured Osmolality: 305 mOsm/kg
Measured pH: 7.8
Note: 20 liters of SHP-1 flush solution was prepared using Dextran-40 as the colloid to minimize cold agglutination. A final flush of 6 liters of ViaSpan organ preservation solution (composition given in Table II) was used for air transport, to minimize the potentially damaging effects to the vascular endothelium of prolonged exposure to Dextran-40.
The use of SHP-1 and ViaSpan, both intracellular perfusates, as a cold ischemic transport medium, is supported by both the clinical and experimental organ preservation literature (references) and in house research where SHP-1 has been used to successfully recover dogs without lasting neurological deficit following 4-hours of asanguineous perfusion with this solution at ~5°C.
Table II
Viaspan Composition

Femoral Cannulation
Femoral cut-down to connect the patient to the extracorporeal circuit for total body washout was begun at 1925. The patient’s esophageal temperature at that time was 13.3°C and the rectal temperature was 15.1°C. The patient’s right groin was prepared for femoral cut-down by scrubbing/swabbing with povidone iodine solution (Betadine) and draping with sterile towels. The anatomical position of the right femoral artery and vein were located by reference to the pubic tubercle and the anterior superior iliac spine. An incision with a #10 scalpel blade was made at the midpoint between these two structures, beginning with the inguinal ligament and running parallel to the longitudinal axis of the leg for approximately 5 cm.
The femoral artery and vein were dissected free and #2 silk ties placed on the proximal and distal exposures of both vessels. The distal ties were tied to achieve occlusion. There was a vigorous arterial pulse with each chest compression from the Thumper, and the capillary blood (ooze from skin and fascia) appeared well-oxygenated.
An arteriotomy was made with a #11 scalpel blade. Upon opening the femoral artery, it was noted that the arterial blood appeared bright red with oxygen saturation estimated visually at >80%. A USCI Type 1966, 18 Fr. arterial cannula was then introduced and secured with the proximal tie. A veinotomy was performed in the same fashion and a USCI type 1967, 26 Fr. venous cannula was advanced until the tip was well within the inferior vena cava and near the heart, and secured with the proximal tie. Venous blood appeared well saturated with oxygen.
The arterial perfusion line was connected to the arterial cannula with a 3/8″ straight connector with port, and the port fitted with a Cobe 3-way stopcock for evacuation of air.
The venous return line was connected to the venous cannula with a 1/2″ straight connector with port, and air was removed from the venous cannula and venous line with a 35 cc plastic syringe.
Blood washout was carried out employing a custom-built, 2-head roller pump, a William Harvey 1500 bubble oxygenator, and a Shiley SAF-20, 20µ blood filter. Perfusion pressure was measured at the Shiley SF-20 filter, anterior to the arterial cannula, employing an aneroid manometer with a sterile Tri-Med Isolator flexible membrane barrier to protect the aneroid from fluid contamination. A calibration curve of measured back-pressure versus measured flow was generated in advance to account for the pressure increase resulting from cannula-induced flow restriction. Temperatures were measured with a YSI 42SL telethermometer. The extracorporeal circuit is presented in schematic below:

TOTAL BODY WASHOUT CIRCUIT
1. Perfusate mixing/holding reservoir (nonsterile)
2. Roller pump (perfusate filtration)
3. Pall 0.2 micron (sterilizing) filter
4. William Harvey 1500 bubble oxygenator
5. Roller pump (arterial)
6. Shiley SAF-20 arterial 20 micron filter
7. Manometer pressure monitoring assembly
8. Bleed line from filter to oxygenator
9. Arterial cannula
10. Venous cannula
11. Dump line
12. Heat exchanger ice water supply
The extracorporeal circuit was primed with 3 liters of washout solution, the composition of which is given in Table I. At the start of blood washout, chest compressions were discontinued and the mask of the Esophageal Gastric Tube Airway (EGTA) was removed (the obturator was left in place to guard against aspiration of gastric contents and water from the PIB). Blood washout was begun at 2010 in the preparation room of the mortuary, with an initial 20 liters of Dextran-40-containing SHP-1 base perfusate at arterial pressures of between 60-80 mmHg, a flow rate of approximately 1600 cc/min. and an oxygen flow rate (to the oxygenator) of 5 liters per minute. The patient’s temperatures at the start of washout were 9.5°Cesophageal and 11.8°C rectal. Perfusion with the 20 liters of SHP-1 was completed at 2030.
Perfusion of six liters of Viaspan was begun at 2034 at a pressure of 60 mmHg and was concluded at 2040. Arterial pump flow rate averaged one liter per minute. A venous effluent sample drawn from the venous line at 2012 near the beginning of the first pass disclosed the following:

A venous effluent sample taken at 1039, at the conclusion of ViaSpan flush revealed the following:

Graphs of laboratory evaluations of samples taken during TBW are presented below:
A-1049 SGOT

A-1049 SGPT

A-1049 BUN

A-1049 Alkaline Phosphatase

A-1049 Glucose

A-1049 Calcium

A-1049 Sodium

A-1049 Potassium

A-1049 Chloride

A-1049 gamma-GT

A-1049 LDH


Photo 9: With CPS continuing, the patient is prepped and draped for femoral cutdown. Surgery to raise the femoral vessels for fem-fem bypass has just begun.

Photo 10: Blunt dissection to isolate and raise the femoral vessels is underway.

Photo 11: The wire reinforced venous cannula has been placed and secured in the inferior vena cava (via the right femoral vein; right side of photo) and the arterial cannula is being placed in the right femoral artery but is not yet de-bubbled or secured.
<

Photo 12: The arterial cannula is being aspirated to remove any trapped air bubbles prior to connecting it to the extracorporeal circuit. Note the bright red color of the arterial blood indicating good gas exchange.

Photo 13: The arterial and venous lines are connected to the arterial and venous cannula immediately prior to the start of extracorporeal support and TBW. Note the bright red arterial blood which has backed up into the arterial line as a result of systemic blood pressure generated by the Thumper.
Remarkably, no cold agglutination was observed during either TBW or subsequent cryoprotective perfusion of this patient. This patient’s Marsh Agglutination Score was 2. The presence of visibly agglutinated masses of red blood cells has been an almost uniform finding in prior cases where ultra-profound hypothermia has been induced with red blood cells (RBCs) present. Even after TBW, agglutinated RBCs are typically observed following air transport when cryoprotective perfusion is subsequently initiated [12].
TBW was concluded at 2040, with an esophageal temperature of 4.6°C and a rectal temperature of 4.9°C. The patient was cleaned up on the mortuary prep table and transferred to a heavy-duty (8 mil) vinyl body bag. At this time (2124) it was noted that there was no rigor present. The body bag containing the patient was then placed atop a bed of Zip-Loc bags containing crushed ice which had been laid down inside an insulated air transport box. The patient was then covered with additional bags of crushed water ice and the transport container was closed for air transport from Sonoma County Airport to Alcor’s perfusion facility in Riverside, California
Air transport was by private, prop-driven aircraft which arrived at Riverside Municipal Airport at approximately 0145 on 10 June, 1990. Air transport was uneventful. The patient was collected from the aircraft and transported to the Alcor facility by Cryovita van, arriving at approximately 0212.

Photo 14: The fibderglass insulated air transport case is prepared to received the patient. A bed of Zip-Loc bags containing crushed ice is lad out on the bottom of the inner metal “Ziegler” container.

Photo 15: The patient, atop the bed of ice bags, is covered over with additional ice bags prior to closure of the air transport container.

Photo 16: The patient loaded aboard a prop-driven aircraft for transport to Alcor’s facilities in Riverside, CA.
A-1049 Cooling Temperatures (Transport)
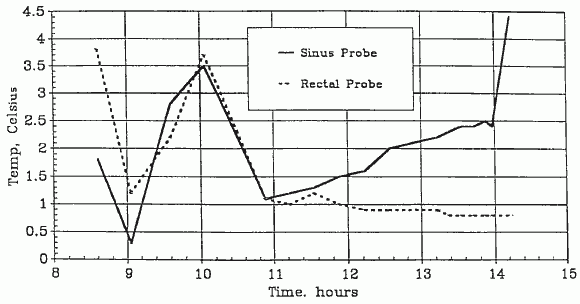
The patient’s arrival temperatures were: 1.8°C esophageal and 3.8°C rectal. At 0423 the patient was transferred from the air shipment container to the weighing surface of an Acme model SRD-2S in-bed where scale the patient’s weight was determined to be 32.8 kg. The patient was then transferred to the operating table, the surface of which had been previously prepared with a cooling blanket (connected to a Cincinnati Subzero BlanketrolTM Hypothermia unit) placed atop a 2″-thick egg-crate foam mattress. The temperature of the coolant passing through the blanket was ~5.0°C After the patient was on the operating table, she was briefly examined. The exam disclosed a profoundly cachectic Caucasian female in her late 60’s. The thorax and limbs were skeletal in appearance, the abdomen was sunken. The eye exam disclosed bilaterally dilated pupils with corneal misting. The lenses of the eyes were opaque due to the reversible, thermally-driven precipitation of the gamma crystallins (-III and -IV) [13]. The globes were somewhat retracted in their sockets. The buccal mucosa was whitish-yellow and apparently blood-free. The skin was pale, uniform in color, and apparently blood free. The skin under the HLR piston was bruised and erythematous in appearance. The distal half of the sternum exhibited the “caved in” green-stick fractured appearance which is typical following extended HLR support.
The patient was free of any signs of rigor mortis and there was no evidence of post-mortem lividity.
Cryoprotective Perfusate Preparation
The composition of the perfusate employed to carry out cryoprotective perfusion is given in Table III. Dry chemical perfusate components were prepared from reagent or medical grade chemicals weighed out using an Ohaus Centogram model 311, and Ohaus Triple Beam 2610 g balances. Dry components were mixed with American Chemical Society (ACS) reagent grade glycerol and/or sterile water for injection USP, or sterile water for irrigation USP. Perfusates were sterilized by filtration into the recirculating and cryoprotective concentrate reservoirs through a Pall PP3802 0.20µ pre-bypass filter. Perfusate was prepared in two batches with the following volumes and glycerol concentrations:
|
Description
|
Volume
|
Glycerol% (w/v)
|
|
Recirculating
|
20 liters
|
5% (w/v)
|
|
Concentrate
|
20 liters
|
86% (w/v)
|
TABLE III
Total Body Washout Perfusate

pH was 7.8 (measured).
mOsm (base perfusate) was 398 (measured).
Cryoprotective perfusate was prepared by dissolving the above components for 40 liters in sufficient water to yield 3 times the above concentrations, dividing the resulting solution into two equal parts, and diluting to the indicated component concentration (making to 20 liters) with water for injection/irrigation USP and glycerol in the appropriate proportions to make 20 liters of solution at the desired glycerol concentration.
Pre-operative Prep
The patient was prepared for a median sternotomy and cranial burr-hole by shaving the head and thorax and scrubbing/swabbing them with povidone iodine solution (Betadine). The sternal operative site was defined by draping with sterile towels and an adhesive operative drape (3M) was placed over the sternum. A cardiac drape was placed over the patient, “tented” on two IV poles at the head, and allowed to extend down over the feet and over the sides of the table by a minimum of 24″. The top of the scalp was draped with a fenestrated adhesive drape over the right frontal lobe.
Median Sternotomy and Vascular Access
Median sternotomy was begun at 0500 on 10 June with an incision over the midline of the sternum with a #10 scalpel blade. Fascia and connective tissue were cleared down to the sternum with an electrosurgical knife. A median sternotomy was then performed with a Sarns sternal saw. The edges of the sternotomy were padded with laparotomy sponges, a self-retaining retractor placed, and the sternotomy retracted open. Blunt and sharp dissection were used to expose the pericardium. The ascending aorta was freed from the pulmonary artery by blunt dissection with Metzenbaum scissors. An aortic cross-clamp was placed just above the aortic valve to exclude the coronary circulation. A second aortic cross-clamp was applied to the descending aorta just distal to the left subclavian artery in order to exclude any arterial circulation to the body.
The left subclavian artery was identified and followed to locate the left vertebral and mammary arteries. Number 2 silk ties were placed on the mammary artery and on the subclavian, just distal to the vertebral, and secured to exclude these vessels. This directed flow to the left vertebral artery, supplying the brain, and excluded the brachial and thoracic wall circulation.
The innominate artery was located and followed to identify the right subclavian artery. The right subclavian was followed to identify the right vertebral and mammary arteries. Silk ties were placed, as was done over the left side, to direct flow to the vertebral artery.
A ventral midline pericardiotomy was made using Metzenbaum scissors. Four stay sutures of 3-0 silk were placed in the margins of the pericardiotomy. These sutures were tied to the sternal retractor, thereby reflecting the pericardium away and creating a pericardial “cradle”, and exposing the heart and aorta for cannulation. A Sarns cardiotomy sucker was used to suction away the pericardial fluid.
A 3-0 TI•CRON™ purse-string suture was placed in the aorta and a snare applied. An aortotomy was made with a #11 scalpel blade. A 22 Fr. aortic perfusion cannula was primed with normal saline and a clamp placed on the distal end. The cannula was then introduced into the aorta and snared in place with a hemostat.
A Satinsky partial occlusion clamp was placed on the right atrium just below the apex. A purse-string suture of 2-0 TI•CRON™ was placed in the atrium and a snare tube applied. An atriotomy was made by removing the apex of the right atrium with Metzenbaum scissors. A tubing clamp was placed on the distal end of the 32 Fr. USCI type 1967 venous catheter, and it was advanced through the atriotomy (with concurrent release of the Satinsky clamp) into the right atrium to the superior vena cava. Umbilical tape was passed around the superior vena cava and tied below the cannula tip. In order to prevent contamination of the recirculating system with venous circulation from the extremities, silk ties were placed on the left and right innominate veins just distal to the left and right internal jugular veins. Venous return was collected from the cannula in the superior vena cava.
A third small purse-string suture of 5-0 silk was placed in the left lateral aspect of the ascending aorta and an aortotomy made with a #11 scalpel blade. A Cobe 3-way stopcock was fitted to an Aloe arterial pressure monitoring catheter, and the catheter was flushed with normal saline and introduced through the aortotomy into the ascending aorta. The catheter was secured in place by applying a snare to the 5-0 suture.
The sterile perfusion tubing was then brought up to the surgical field and secured in a Travenol tubing holder towel-clamped to the drapes. The arterio-venous loop of the perfusion circuit was clamped and divided by cutting out the 1/2″ – 3/8″ adapter with Mayo scissors. A 1/2″ connector with a Cobe 3-way stopcock was used to connect the 1/2″ ID venous return line to the venous cannula. Air was cleared from the system with a 100 cc glass syringe. A Cobe 8 ft. pressure monitoring line was fitted to the arterial pressure catheter, flushed with normal saline, and handed off the field to be connected to a Trantec Model 800 pressure transducer and a Tektronix Model 414 monitor.
Surgery to connect the patient to the perfusion circuit was completed at 0740.
Cranial Burr-Hole
Surgery to open the cranial burr-hole was begun at 0527. The vertex of the scalp approximately 3 cm to the right of midline over the right frontal lobe was incised with a #10 scalpel blade and an incision approximately 4 cm long was made down to the periosteum. A periosteal elevator was used to expose the bone approximately 3 cm to the right of the midline. A 10 mm hole was made with a Hudson Brace burr and drill. The dura mater was opened and trimmed away with iris scissors to expose approximately 6 to 8 mm of the cortical surface. The burr hole was opened at 0550; the pial vessels, including a large pial vein directly under the burr hole were noted to be free of blood and the cortical surface appeared pearly white. The cortical surface was 2 mm below the cranial bone, indicating slight cerebral dehydration, probably secondary to the patient’s dehydrated state at the time of cardiopulmonary arrest and subsequent perfusion of hyperosmolar solutions during TBW.
Cryoprotective Perfusion Circuit
The extracorporeal circuit for cryoprotective perfusion is shown in schematic below:

CRYOPROTECTIVE PERFUSION CIRCUIT
1. Cryoprotective concentrate reservoir
2. Recirculating reservoir
3. Arterial roller pump
4. Shiley Tamari-Kaplitt pulsator
5. Sci-Med 1.8 sq. meter oxygenator
6. Sarns Torpedo pediatric heat exchanger
7. Pall EC1440 40 micron arterial filter
8. Stopcock (bleed line)
9. Stopcock (air removal)
10. Arterial (aortic) cannula
11. Venous cannula
12. Venous sample line
13. Cryoprotective withdrawal/addition roller pump
14. Withdrawal line to drain
15. Cardiotomy sucker
16. Cardiotomy suction pump
17. Bleed line from filter to recirculating reservoir
18. Stirring table (mixer for recirculating reservoir)
The circuit consisted of two parts: a recirculating system to which the patient was connected, and a cryoprotectant addition system which was connected to the recirculating system. The recirculating system was a 20 liter high density polyethylene reservoir sitting atop a magnetic stirring table, an arterial (recirculating) roller pump, a Sci-Med 1.82 meter oxygenator, a Sarns Torpedo heat exchanger and a Pall EC1440 40 micron blood filter. The recirculating (mixing) reservoir was continuously stirred with a 2″ Teflon-coated magnetic stirring bar driven by a Thermolyne type 7200 magnetic stirrer. The cryoprotectant addition system consisted of a 20-liter high density polyethylene reservoir containing 86% (w/v) glycerol (see Table I) and a Drake-Willock model #7401 hemodialysis pump running two 1/4″ tubing lines which acted as: 1) A withdrawal pump which removed perfusate from the recirculating system, and 2) An addition pump pumping 86% (w/v) glycerol perfusate from the concentrate reservoir into the recirculating reservoir.
Arterial and venous samples for evaluation of chemistries and glycerol concentration were drawn at 15-minute intervals during cryoprotective perfusion. Arterial samples were drawn from a 3-way stopcock interposed between the arterial filter and the filter vent line. Venous samples were drawn from an 8′ Cobe monitoring line connected to a Cobe 3-way stopcock attached to the venous connector connecting the venous cannula and the venous return line. (The dead-space of the Cobe monitoring line was determined and this volume was drawn up and discarded before each sample was taken.)
The perfusion circuit was prepared in advance of need and was sterilized with ethylene oxide using an appropriate protocol of post-sterilization out-gassing and aeration. Nitrogen gas delivered to the oxygenator at a flow rate of 15 liters per minute was used throughout cryoprotective perfusion to reduce the possibility of oxygen-mediated reperfusion injury following prolonged cold ischemia.
Cryoprotective Perfusion
Closed-circuit perfusion of 5% (w/v) glycerol perfusate was begun at 0744 but had to be immediately discontinued due to bulging of the cerebral cortex +1-2 mm into the burr hole. The problem was resolved by advancing and rotating the venous cannula in the superior vena cava. Perfusion resumed at 0801 at a flow rate of 500 cc/min., sinus temperature of 5.2°C, arterial temperature (perfusate) of 6.3°C and a mean arterial pressure (MAP) of 50 mmHg. At 0805 arterial and venous pH and gases were as follows: arterial pH 7.58, arterial pO2 35, arterial pCO2 10.3, venous pH 7.46, venous pO2 48, and venous pCO2 20.8. The glycerol ramp was begun at 0801 at a flow rate of 160 ml/min.
Pulsatile flow was initiated at 0810 using a Tamari-Kaplitt pulsator at a rate of 60 pulses per minute. Pulse pressure was adjusted to 100/10 mmHg and the flow rate was gradually increased from 500 ml/min. to a peak of 850 ml/min. At the start of pulsatile perfusion until well into the glycerol ramp at approximately 09:00 the cortical surface was observed to pulsate; rising and falling with each pulse. This phenomenon is commonly observed in patients with beating hearts during neurosurgical procedures but had not been previously observed in a human cryopreservation patient subjected to pulsatile flow. Cortical pulsation during pulsatile perfusion in the cryopreservation patient appears to be dependent upon at least two factors: absence of elevated intracranial pressure (i.e., no cerebral edema) and a compliant cerebral vasculature which is free of vasoconstriction or rigor.
At 0820 glycerolization of the face and scalp was noted to be very uniform with no patchy non-perfused areas noted. Circulation through the scalp and dura was judged to be excellent with the only exception being the skin at the margins of the craniotomy incision where it was compressed by the prongs of the Weitlaner retractor. At this time drainage from the burr hole had increased from 15cc-20cc/min to a rate of 150-200 ml/min. This drainage of perfusate from the burr hole is presumably as a result of leakage from the scalp wound, cranial bone, and incised dura and increases in severity as tissue cryoprotectant concentration rises. (Note: It has since been determined that the most likely cause of perfusate leakage from the burr hole is as a result of fluid leakage from tears or ruptures in the bridging veins between the dura and the arachnoid as a result of shrinkage of the cerebral hemispheres in response to glycerolization [14].
Cryoprotective Ramp
The recirculating perfusate withdrawal/glycerol concentrate addition flow rate was set at 160 ml/min., yielding a 50 mM/min rate of increase in arterial glycerol concentration. This resulted in an average arterial/venous difference in glycerol concentration of approximately 550 mM over the course of cryoprotective perfusion.
The initial response to the start of the cryoprotective ramp was good, with cerebral cortical volume rapidly decreasing to 2-3 mm below the margin of the burr hole. The brain continued to shrink until the cortical surface was estimated as being 6 mm below the calvarium. The brain appeared caramel colored and shrunken within the burr hole at the conclusion of cryoprotective perfusion. This is an appropriate response to high molarity glycerol perfusion indicative of both cellular and interstitial dehydration and more importantly the absence of edema in either tissue compartment.
Cryoprotective perfusion was concluded at 0945 at an arterial flow rate of 488 ml/min., arterial temperature of 6.0°C, sinus temperature of 6.2°C, and recirculating withdrawal/CPA addition rate of 164 ml/min.
The final venous cryoprotectant concentration was 4.5 M as measured by freezing point depression osmometery using a Precision Systems Osmette A osmometer.
The concentration of glycerol in the arterial and venous effluent, the arterial and esophageal temperatures, arterial pressure, arterial flow rate and arterial and venous pH and gases are shown graphically on the following pages:
A-1049 Glycerol

A-1049 Cooling Temperatures (Perfusion)

A-1049 Mean Arterial Pressure (Perfusion)

A-1049 Arterial Flow rate (CPA Perfusion)

A-1049 pH (CPA Perfusion)

A-1049 pO2 (CPA Perfusion)

A-1049 pCO2 (CPA Perfusion)


Photo 17: The cryoprotective perfusion circuit is strung on the Sarns heart-lung machine. Above the perfusionist’s extended right arm is a Tektronix invasive pressure monitor and a Physiotemp multiple channel thermocouple temperature monitor. Barely visible in the foreground behind the red biohazard bag is the Tamari-Kaplitt pulsatile flow device.

Photo 18: The cryoprotective (CPA) perfusion circuit has been primed and readied for the start of CPA perfusion. The Sci-Med membrane oxygenator is visible at the lower left of the photograph.

Photo 19: Median sternotomy and surgery to connect the patient to the CPA perfusion circuit is underway. The aortic root and right atrium are used as vascular access sites for cephalic perfusion.

Photo 20: CPA concentration, arterial and venous blood gases and pH are analyzed in real time in the operating room.
At 0945, the silastic-coated tip of a 15′ long, 30 gauge, Kapton-wrapped copper-constantan (type T) thermocouple probe (Instrument Laboratory #53-30-513) was threaded into the burr-hole and placed on the cortical surface. The burr-hole was then filled with bone wax and the scalp closed with surgical staples. The suture line was protected with Parke-Davis spray-on bandage and the probe wire was anchored to the scalp with surgical staples. Brain surface temperature was measured at 6.7°C at 0957.
Cephalic Isolation
Surgery for cephalic isolation was begun at 1003 with a circumferential skin incision made at the base of the neck and extended anteriorly and posteriorly to just below the margins of the clavicle. The skin was dissected free from the underlying connective tissue up to the level of the 5th cervical vertebra to form skin flaps. The cervical musculature and other anatomical structures were then severed with a #10 scalpel blade down to the junction of the 5th and 6th cervical vertebrae. A Gigli saw was then passed under the vertebral column and the cut was made at approximately the level of the 5th cervical vertebra, which freed the head from the body.
The cervical skin and musculature were observed to be dark in color, evenly stiff, and waxy in texture, consistent with uniform glycerolization. The spinal cord was observed to be somewhat shrunken within the vertebral foramen.
Skin flaps were then closed over the stump of the neck using a skin stapler, after the edges of the flaps were first approximated using interrupted 2-0 TI•CRON™ suture. Cephalic isolation was completed at 1014.
Cooling to Dry Ice Temperature
Temperature descent to -77°C was monitored with probes in the frontal sinus, the brain surface, and head surface (placed temporally) and an additional probe was used to monitor bath temperature. Bath, external, and sinus probes were Instrument Laboratories 53-20-507, “load type”, 20 gauge, Teflon-coated copper-constantan thermocouples. (The. 53-20-507 TC probe placed in the frontal sinus was used to replace the clinical TC probe employed to monitor pharyngeal temperature during perfusion.) TC probes were anchored into place with surgical staples.
The patient (cephalon) was placed inside two 2 mil polyethylene plastic bags and lowered into a 15 liter bath of 5 centistoke polydimethylsiloxane oil (Silcool) which had been pre-cooled to -11.2°C. The first temperature readings taken at 1025 were: brain surface 3°C, sinus 5°C, temporal (skin surface) -5°C, and bath -10°C. Cooling to -77°C was at a rate of approximately -4°C per hour to -44°C and 15°C per hour to -77°C. A temporal to sinus temperature differential of approximately 16°C was maintained during cooling to -40°C. The temporal to sinus temperature differential was increased to 18°C during cooling to -77°C. Cooling to -77°C was complete by 2400 on 11 June, 1990. The patient’s dry ice cooling curve is presented below. Times shown are in decimal hours post-arrest.
An esophageal TC probe was not placed in the patient and as a consequence true surface to core temperature differential could not be measured. A decision was made to cool the patient at a surface to frontal sinus temperature differential of ~16°C to the eutectic point of glycerol-water-sodium chloride solution which is ~ -64°C. This approach yielded a cooling rate of ~ 3.0°C/hr to -40°C and a cooling rate of ~4.0°C/hr to -70°C.
A-1049 Cooling Temperatures (Dry Ice Bath)

A-1049 Surface – Core Difference (Dry Ice Bath)

On 23 March, at 2256 the patient was removed from the Silcool bath, the outer Silcool-wetted plastic bag was stripped off, and the patient was placed inside a polyester pillow case resting on a bed of Dacron wool at the bottom of an aluminum neurocan. The pillow case was then closed with a white cotton shoe lace to which was affixed a stainless steel tag identifying the patient. The neurocan was then nested inside a 5 gallon plastic pail on a bed of crushed dry ice and then surrounded with crushed dry ice on all sides to the top of the open neurocan. The neurocan and packaging had been pre-cooled to ~-90°C by spraying with liquid nitrogen. The neurocan lid was then placed on top of the neurocan and the neurocan was covered over with additional crushed dry ice. The pail was then suspended by a nylon cord attached to a pulley and lowered into a Bigfoot whole body dewar (see accompanying diagram). Temperature descent was controlled by lowering (or, if necessary, raising) the pail with the neurocan (and dry ice thermal ballast) towards a pool of approximately 300 liters of liquid nitrogen in the bottom of the whole body dewar.

As can be seen from the graphs above, control of cooling using this method was unsatisfactory. Temperature descent occurred more rapidly and far less uniformly than desired with one excursion in sinus to brain surface temperature differential of 10 °C occurring between 60 and 80 hours post arrest. Between 100 and 120 hours post arrest the patient’s external temperature was rapidly decreased from -150°C to ~ -190°C resulting in a decrease in sinus temperature of 15°C in ~ 1 hour. The purpose of this maneuver was to hopefully induce fracturing events at relatively high temperatures below Tg on theoretical grounds that a smaller number of comparatively large fractures is preferable to a large number of small fractures. Fracturing is known to occur at some point in human cryopatients cooled to below the glass transition point Tg of the patient [15]. In aqueous cryoprotectant-water solutions the closer fracturing is initiated to Tg the fewer the number of fractures.
Allowing fracturing to be delayed until the solution is cooled far below Tg results in a very large number of small fractures distributed throughout the solution. The intention was induce fracturing at ~-120°C in this patient. However, the patient cooled more rapidly than expected and had a reached a temperature of -150°C before fracture initiation could be carried out. A decision was made not to rewarm the patient, but rather to attempt to initiate fracturing before the patient’s temperature decreased further.
In the future computer controlled gas convection cooling would seem highly desirable. In the absence of this, a return to the use of a heavily insulated container to hold the neurocan as been done in previous cases, would seem to be indicated. Good insulation provides for slower and much more uniform and easily controlled cooling. It also virtually eliminates the danger of rapid and extreme temperature excursions during both cooling and subsequent transfer of the patient to long term storage.
At 0036 on 12 June, 1990 the cooling assembly containing the patient was lowered into a Bigfoot dewar to which approximately 300 liters of liquid nitrogen had been added. The patient was lowered to a stratum in the dewar where the ambient temperature was approximately -130°C. Thermocouple probes were led out of the Bigfoot dewar and connected to an Omega 2165A thermocouple thermometer. The neurocan was then raised or lowered as needed to obtain the desired rate of temperature descent. Cooling to -196°C was achieved at 2400 on 15 June, 1990. The patient was placed into long-term cryogenic storage at 0044 on 20 June by submersion in liquid nitrogen in an MVE A-2600 cryogenic dewar. The patient’s liquid nitrogen cooling curve is presented below.
A-1049 Cooling Temperatures (LN2)

A-1049 Surface – Core Difference (LN2)

Renal Viability Evaluation
During the course of cryoprotective perfusion the patient’s abdomen remained packed in ice in order to maintain the temperature of the abdominal viscera at 1-2°C. At the conclusion of cryoprotectant perfusion the patient’s left kidney was removed via a mid-ventral laparotomy and transferred to a screw-cap, liquid-tight polypropylene laboratory bottle containing ~300 cc of ViaSpan pre-chilled to 2°C. This container was then placed inside a Zip-Loc plastic bag which was in turn placed inside an insulated container which was filled with flaked water ice. The kidney was then air-shipped via Federal Express to a cryobiology research laboratory where it was subjected to viability analysis using the tissue slice intracellular/extracellular sodium/potassium ratio technique [1].
The patient’s average renal Na+/K+ ratio was 3.5 +or – 0.27 which is the expected value for a tissue storage interval of approximately 2.5 days. It should be noted that this Na+/K+ ratio is remarkable considering that the assayed organ was removed from an elderly woman whose proximate cause of cardiac arrest was malnourishment and dehydration complicated by a prolonged agonal period in deep shock. A renal Na+/K+ ratio of < or = to 3.5 is typical for non-ischemic rabbit kidneys stored for 48 hours in the laboratory where the analysis was performed. Further, the response of the patient’s renal cortex to cryoprotectant toxicity studies closely paralleled studies conducted with fresh rabbit renal cortex in the same laboratory. The Na+/K+ ratio for freshly harvested kidneys in the evaluating laboratory is typically between 4.5 and 5.5. Serum/Perfusate Electrolytes and Enzymes
Laboratory evaluations of samples taken during cryoprotective perfusion are presented in full in both graphic and tabular form as an addendum to this document below.
Sudden Death Risk Assessment
This case demonstrates the importance of a thorough assessment of the patient’s medical condition at the time of terminal diagnosis and any associated risks of sudden death with appropriate mitigating medical intervention. This patient’s history of coronary atherosclerotic disease coupled with neoplasia associated hypercoagulability resulted in potentially life threatening arterial and venous thromboembolic disease. The high incidence of hypercoagulable states associated with malignant disease, particularly in the elderly, and the accompanying increased incidence of sudden death from deep vein thrombosis, pulmonary embolism, heart attack and stroke should be given careful consideration in the future [16-21]. Where feasible and economically possible, it would seem advisable to perform laboratory studies for hypercoagulability at the time of terminal diagnosis and at intervals during the antemortem period. In general, patients at risk of hypercoagulability (most cancer patients) and resultant risk of thromboembolic disease should consider prophylaxis with 800 IU/day of vitamin E and sufficient aspirin to acetylate platelets without unduly elevating the risk for gastrointestinal side effects; a dose of 300 mg/day [22].
Dehydration: Practical and Clinical Considerations
This cryopreservation patient’s decision to voluntarily end her life via dehydration was not the first [23] and will probably not be the last. In both this case and the previous case the clinical course was much the same with the patient experiencing one or two short periods of agitation, confusion and delirium after the first few days of reduced hydration. This alteration of sensorium in combination with no urine output triggered anxiety in the family and the hospice personnel that the patient’s cardiopulmonary arrest was imminent. Anuria is a very real indicator of imminent cardiac arrest in critically ill and dying patients and may be used as guide, in combination with other clinical findings, for when to initiate a Standby. As this case demonstrates it is not possible to determine true anuria (cessation of renal urine output) unless the bladder is catheterized and urine output is continuously monitored. In fact, as previously noted, this patient voided 200 cc of urine on her last day of life.
An important rule learned from this case is that anuria may only be accurately determined, and thus used as an indication to start a Standby, when an indwelling bladder catheter is present and continuous urine output can be evaluated over a reasonable time course (i.e., at least 24 hours).
In the absence of a Foley catheter perhaps the next best way to determine the patient’s fluid status and possible time course to cardiac arrest is to measure serum osmolality. A serum osmolality of ~380 mOsm/kg results in stupor or obtundation and a serum osmolality of ~420 mOsm/kg is rapidly (<24 hours) fatal [24].
It is instructive that this patient’s baseline serum osmolality (collected within 5 minutes of cardiopulmonary arrest) was 358 mOsm/kg, well below the numbers reported as life threatening, or imminently lethal in the literature.
When using serum osmolality (or specific gravity) to evaluate a patient it is important to understand that it is primarily a marker for life threatening or lethal levels of dehydration and not likely to be the direct mechanism responsible for cardiopulmonary arrest. The primary etiologic mechanism in death by dehydration in the hospice setting is hypovolemic shock. Disturbances in electrolytes, metabolic derangements directly as a result of hyperosmolality, and blood hyperviscosity can be expected to contribute to, and occasionally directly result in cardiopulmonary arrest. Blood hyperviscosity (and accompanying increased risk of throembolic disease) will be of special concern in patients with an elevated hematocrit.
In the field setting it will likely be impossible to measure serum osmolality, however it should be possible to measure serum specific gravity (SG)(if a blood sample can be obtained) using a hand-held urine/serum refractometer which it is recommended be added to the Standby Kit. ). In most cases, the serum specific gravity varies in a relatively predictable way with the osmolality, with the specific gravity rising by.001 for every 35 to 40 mOsm/kg increase in osmolality. Thus, a serum osmolality of 280 mOsm/kg (which is isosmotic to plasma), is usually associated with a specific gravity of 1.008 or 1.009 [25].
In the event blood samples cannot be obtained due to lack of vascular access or for medicolegal reasons, urine osmolality may serve as a rough guide in assessing the degree of dehydration. In a study of water deprivation in dogs of 96 hours duration the mean maximal urine osmolality was 2,289 mOsm/kg and the corresponding mean maximal urine specific gravity was 1.062 and ranged from 1.050 to 1.076. The ratio of mean maximal urine osmolality to mean serum osmolality at the time of peak urine concentration was 7.3 [26]. A urine osmolality of ~2,200 was associated with severe weakness and obtundation. If these data are applicable to humans a urine specific gravity of 1.050 or greater should be prognostic of terminal dehydration (~24 to 72 hours time course to cardiopulmonary arrest).
In the absence of a refractometer a Squibb Urinometer (a small, low volume hydrometer) may be used to measure urine specific gravity.
In the future, daily measurement of the patient’s weight may prove valuable even if it cannot be continued for long. In fact, inability of the patient to stand due to orthostatic hypotension is a valuable indicator that dehydration is progressing and beginning to have the anticipated negative hemodynamic effects.
Agonal Vitals Trending
This case also demonstrates the importance of regular charting and graphing of vital signs in predicting the patient’s agonal course. Real-time graphic charting of these parameters is critical as it shows trends which are more important than any single reading. It is recommended that a comprehensive flow sheet be developed, similar to those used in ICUs, to graphically display HR, BP, MAP, RR, SpO2 (where available), fluid intake and output, serum/urine specific gravity, GCS, and pupil size and reactivity. Graphic charting of a daily weight (where possible) will also be particularly important in patients undergoing dehydration and in patients with renal failure who are experiencing fluid gains. It is strongly recommended that standardized, pre-printed, data collection sheets for use during the agonal period of Standby be prepared for use in future cases and be deployed with personnel whom are thoroughly trained in their use.
Minaturization and reduction in the cost for pulse oximetery equipment in the near future should allow for economical in-field measurement of patient SpO2 during the agonal period as well as during CPS. This should allow for more reliable prediction of the time course to cardiopulmonary arrest as well as for more definitive evaluation of the effectiveness of CPS in the field in real time.
Over-Ventilation and Alkalosis During CPS
As is evident from the patient’s baseline venous blood sample at the start of CPS there was marked respiratory alkalosis with a pH of 7.65. This was undoubtedly due to over-ventilation from the fixed-setting Thumper ventilator. At this time there seems little alternative to this problem due to the unavailability of in-field arterial blood gas measurement equipment and the inevitable deterioration of the patient’s gas exchange status as a result of CPR-mediated pulmonary edema. Future Thumpers may better employ incorporated time and volume cycled ventilators in place of the fixed-time ventilators currently in use. The ability to adjust tidal and minute volume by titrating ventilation to the patient’s measured EtCO2 is highly desirable. Over-ventilation causes depletion of pCO2 with accompanying cerebral vasoconstriction and reduced cerebral blood flow [27,28].
Not surprisingly the patient was markedly dehydrated at the time of cardiac arrest as evidenced by a baseline serum osmolality of 358 mOsm and a BUN of 47. SGOT, SGPT, LDH and GGT were all elevated at the time of cardiac arrest presumably as a result of both the malignancy and the ante-mortem agonal period with its associated lengthy and profound shock.
Evaluation of External Cooling
Arguably, the most critical initial protective strategy against ischemic injury in the human cryopatient during transport is the rapid induction of profound cerebral and systemic hypothermia [29-31]. From a theoretical standpoint the most effective means of achieving rapid and profound reduction of core temperature would be via extracorporeal cooling [32]. However, due limitations of cost, logistics and surgical time required to achieve vascular access the use of extracorporeal cooling as a first-line modality is likely to remain un-achievable for the foreseeable future. This means that other methods such as external cooling via the application of ice, core cooling utilizing peritoneal lavage with fluids nears 0°C (reference), or a combination of the two are likely to remain the methods of choice for initial induction of hypothermia following cardiopulmonary arrest.
The PIB was developed after in-field core temperature measurements during the transport of a human cryopreservation patient employing ice bags to facilitate external cooling disclosed that this method was grossly unsatisfactory resulting in a core cooling rate of approximately 0.05°C/min during the first 4 hours of cooling [33-36]. Subsequent cases confirmed very slow rates of cooling using externally applied plastic bags filled with water ice ranging between a high of 0.12°C/min to a more typical 0.064°C/min [33].
The PIB was developed by the author to allow for direct contact of ice water with essentially the entire surface area of the patient to simulate rates of heat exchange presumably encountered in cold water drowning where recovery of adults without neurological deficit after 20-40 minutes or more of submersion in ice water has been repeatedly clinically documented [37-40].
Determining the rate of cooling likely to be achieved with the PIB, particularly with the addition of convection cooling by stirring or circulating the PIB water around the patient was important prior to expending the considerable resources required and logistic difficulties to be overcome if these techniques were to be implemented routinely during Transports. A careful examination of the literature was undertaken by the author circa 1987 to determine if there were any published data on the rate(s) of core cooling likely to be feasible with these modalities.
Unfortunately, the only published data were those of the SS (Schutzstaffel) Nazi physicians Holzloehner, Finke, and Rascher, et al., conducted in 1942 on prisoners in the German concentration camp Dachau as part of an effort to understand the mechanisms and time course of hypothermia (as well as methods of safe re-warming) in Luftwaffe pilots downed in cold North Sea water. Most of the actual work was conducted by Sigmund Rascher, M.D. and was summarized after its post-war recovery by Major Leo Alexander of the U.S Army Medical Corps in 1946 [41]. This document, now referred to as the “Alexander Report,” contains detailed information in both graphic and tabular form on the effects of convective cold water external cooling on human prisoners.
At this time, the citation and use of this data are extremely controversial and their relevance, integrity, and scientific usefulness have been called into question [42]. There is no question in the author’s mind that the work of Holzloehner, et al., constitutes an atrocity and a crime against humanity of the worst kind. One of the principal criticisms of the scientific validity of this work has been the observation that many of the more than 300 victims of this research were cachectic and weakened from malnutrition and frank starvation making their physiological responses non-representative of that of the healthy German aviator. Ironically, it is just this criticism that makes the data obtained in this study uniquely valuable to human cryopreservation.
The author, and others in authority at Alcor (principally Alcor Directors and staff scientists Jerry Leaf and Hugh Hixon), had the difficult task of determining whether it was both ethical and scientifically valid to use these data. After extensive discussion and consideration it was decided that it was both ethically and scientifically justified.
Data from the Alexander Report must be interpreted with caution and within the context of both the typical conditions under which human cryopreservation takes place and more recent data on the effects of cold water immersion on human subjects which is unquestionably both ethically and scientifically sound [43,44].
Several important caveats apply: Cooling curve data obtained by Holzloehner, et al., must be evaluated with the understanding that non-paralyzed, conscious individuals subjected to immersion in water cooled to between 2°C and 12°C will shiver either until exhaustion or until the temperature of the skeletal muscle drops below 30°C at which point shivering is impossible [45]. Until either or both of these events happen core temperature will either transiently increase or will not decrease appreciably. Thus, use of these data to evaluate the efficacy of convective cold water immersion must subtract the first 10 to 20 minutes of core temperature data when shivering and elevated oxygen consumption [46] are effectively maintaining homeostasis in the face of profound external cooling.
Having said this, it is interesting to note that Holzloehner, et al., found no difference in the overall rate of cooling in anesthetized versus un-anesthetized subjects. This argues for the continued use of drugs to induce paralysis via neuromuscular blockade and thus inhibit both fine and coarse twitch shivering.
From the graphic data it is very apparent when this point is reached, typically at about 15 minutes post-immersion, for the unclothed emaciated subject. At this point the cooling curve begins to decline markedly and within 5 minutes achieves a steady rate of descent of approximately 0.26°C/min to 28°C after which it slows appreciably to 0.13°C/min until cardiac arrest typically occurs at ~27°C. This slowing is probably due to deteriorating hemodynamic status (BP was typically 40 mmHg to 50 mmHg near the terminal portion of the cooling curve) and a decrease in the ∆T between subject core temperature and bath temperature. In one series of 7 subjects the mean time from immersion to ventricular fibrillation (VF) was 66 minutes with a mean rectal temperature at the point of VF of 26.94°C. The fastest rate of cooling observed was 0.36°C/min in malnourished females.
One potentially very important observation made by of Holzloehner, et al., was the lack of any difference in the rate of early core temperature drop between subjects immersed in stirred water baths at 2°C or 12°C. This would seem to imply that the rate limiting factor in cooling, at least to ~27°C, is not the volume of water flowing over the subject nor the ∆T so much as peripheral vasoconstriction resulting in decreased blood flow to tissues in contact with cold water. Stirring of the PIB water in excess of that required to achieve optimum heat exchange is also undesirable for the following reasons:
- Given the modest amount of stirring employed in the experiments of Holzloehner, et al., it is unlikely that movement of large volumes of water over the patient will increase the rapidity or efficiency of heat exchange.
- In this case a 1/3 horsepower sump pump moving 40 gallons of water per minute, drawing 4 amps, and generating 500 watts total mechanical and waste heat is excessive. While 500 watts will melt only 6 kg of ice per hour, the movement of this much water across the un-insulated surfaces of the PIB will result in much of the heat absorbing capacity of the ice being used to cool ambient air and the PIB base-plate instead of the patient.
- Smaller more efficient pumps will allow for the use compact and portable deep-cycle batteries facilitating initiation of SCCD cooling in the field and during vehicular transport where 110VAC wall current is unavailable.
- Increasing the efficacy of external cooling further will require improvements to either or both cardiac output and peripheral perfusion by either pharmacologic and/or mechanical means.
It is important to consider that temperature was measured rectally in the subjects of these experiments as opposed to esophageally or tympanically and thus cardiac and brain core temperatures were probably 2°C to 4°C lower than those reported since rectal temperature is known to lag significantly behind esophageal temperature during induction of hypothermia during both external cooling and extracorporeal cooling [47-49].
Third, comparison between subjects is difficult due to the lack of body mass measurements or any determination of fat cover; this is a problem which must also be resolved in human cryopreservation cases to facilitate comparisons of the efficacy of cooling methods among cases.
Finally, quantification of hemodynamic status during cooling was not done. Blood pressure was measured only after the subject was removed from the cooling bath; not during active cooling. Similarly, measurement of cardiac output and peripheral and systemic vascular resistance were not techniques available at that time. However, it seems reasonable to presume that as the investigators reported, most subjects were maximally peripherally vasoconstrcited. This can be inferred from the gruesome clinical descriptions contained in the data which report facial skin becoming immediately pale and then cyanotic after the first 45 to 50 minutes of cold water immersion (i.e., near the point of cardiac arrest).
The data also disclose the importance of cooling both the neck and the occiput of the head if the maximum rate of temperature descent was to be achieved, presumably reflecting both the rich perfusion of the scalp and the cooling of the high flow and relatively superficial jugular and carotid blood flows. Failure to cool the neck and occiput were sufficient to reduce core temperature drop by approximately 0.16°C/min.
The data contained in the Alexander Report were sufficiently convincing for the author to initiate a change in Alcor protocol from ice bag cooling to one employing cold water immersion using the PIB. This decision was financially costly and introduced serious new logistical hurdles. PIBs must be constructed, air transported, and add hundreds of additional pounds of weight in water and ice which must be picked up and moved necessitating additional personnel.
Late in 1989 Fred Chamberlain, co-founder of Alcor and an Alcor certified Transport Technician, developed a prototype SCCD using an AC powered sump-type submersible pump to facilitate convective cooling which he called the “squid” because of its configuration.
This was the first time that the SCCD has been used. It is difficult to evaluate the effectiveness of this new modality for the following reasons:
- The patient was profoundly cachectic with a mass of only 32.8 kg and virtually no fat cover.
- The patient exhibited an extraordinarily good and sustained response to CPS maintaining excellent peripheral tissue perfusion and oxygenation throughout the entire 2.5 hours of Thumper supported CPS.
- The patient was completely bald.
Given the caveats above, a core cooling rate 0.41°C/min during the first 12 minutes of external CPS and external cooling and of 0.33°C/min during the first 43 minutes of external CPS and external cooling still appears extraordinary for cryopreservation patients although it is consistent with the data for cachectic human concentration camp victims generated by Holzloehner, et al. In the future comparisons between patients can perhaps further be facilitated by using the patient’s Body Mass Index (BMI) as an objectifying comparison tool. BMI is calculated as follows:
BMI=KG/m2
The patient’s weight and height are required to calculate BMI. Thus, it will be critical to measure the patient’s weight in kg (upon arrival at the cryoprotective perfusion facility) and measure their height in cm prior to the start of cryoprotective perfusion.
It may also be very useful to estimate body fat and more reliably determine subcutaneous fat cover by using a Skinfold Caliper. A Skinfold Caliper is a device which measures the thickness of a fold of skin with its underlying layer of fat. By measuring skin fold thickness at precise places on the thorax, abdomen and thigh a reasonably accurate measurement of total body fat can be obtained in a healthy individual. In the case of the cryopreservation patient the interest is not primarily in the accuracy of the tool in determining total body fat per se, but rather in the creation of a database of measurements which can serve as an objectifying guide to determining a patient’s degree of fat cover at the time of cardiopulmonary arrest.
Illustrated in the charts and tables below is the procedure for taking Skinfold Caliper measurements in both adult males and females:
Measuring Sites for Male
|
||
| Site | Direction of Fold | Measurement |
| Thorax | Diagonal | Fold is taken ½ the distance between the anterior auxiliary line and nipple. |
| Abdomen | Vertical | Fold is taken vertical 2 cm lateral to the umbilicus. |
| Thigh | Vertical | Fold is lifted on anterior aspect of thigh midway between inguinal crease and proximal border of patella. Body weight is shifted to left foot. |
Tips on Measuring
|
||
Measuring Sites for Female
|
||
| Site | Direction of Fold | Measurement |
| Triceps | Vertical | Fold is taken midway between the shoulder and elbow joint, on the center of the back of the arm. |
| Waiste | Diagonal | Fold is taken diagonally above the iliac crest along the anterior auxiliary line. |
| Thigh | Vertical | Fold is lifted on anterior aspect of thigh midway between inguinal crease and proximal border of patella. Body weight is shifted to the left foot. |
Multiple regression equations exist for calculating the percentage of body fat from the Skinfold Caliper measurements depending upon age and sex [50-52]. However, at this time the author believes that the selection of a particular algorithm is not very important. Because there is no typical cryopreservation patient, the consistent collection of reliable data and their reduction to arbitrary numbers which can be compared from case to case is the most important consideration.
Collection of BMI and Skinfold Caliper measurements from multiple patients combined with some objectification of the efficacy of CPS during transport will likely be the most useful tools for comparing various methods of cooling; both external and internal.
Below are the Jackson, et al., equations for calculation of percentage body fat in females and males:
FEMALES:
D = 1.0994921 – (0.0009929 x sum of triceps, suprailiac, thigh) + (0.0000023 x square of the sum of triceps, suprailiac, thigh) – (0.0001392 x age), based on a sample aged 18-55. Jackson, et al. (1980) Generalized equations for predicting body density of women. Medicine and Science in Sports and Exercise, 12:p175-182.
MALES:
D = 1.10938 – (0.0008267 x sum of chest, abdominal, thigh) + (0.0000016 x square of the sum of chest, abdominal, thigh) – (0.0002574 x age), based on a sample aged 18-61. Jackson, A.S. & Pollock, M.L. (1978) Generalized equations for predicting body density of men. British J of Nutrition, 40: p497-504.
If further cases, animal research, or both, bear out the utility of using the SCCD it will be desirable to avoid interruption of SCCD cooling during patient movement because of dependence on AC power. Because the work of Holzloehner, et al., seems to indicate that modest convection is effective at the maximum physiologically acceptable ∆T of 32°C to 35°C it should be possible to substitute the AC powered pump used in this case for a small DC powered pump similar to those used in marine bilge applications. These compact, low power consumption pumps typically produce a flow at 3’of head of 2 to 5 GPM and could be easily powered for ~1 hour by a compact deep cycle marine outboard motor battery or even a conventional motorcycle battery. Alternatively and/or supplementally, DC power could be drawn via the cigarette lighter power point to operate the SCCD pump during vehicular transport.
If it is determined that a high capacity AC pump is required, it is suggested that a compact, portable DC to AC inverter might be used to power the SCCD pump using the vehicle’s alternator/battery power supply.
Temperature Data Acquisition
A recurring problem which surfaced in this case is the problem of reliably acquiring patient temperature data at regular intervals during transport. Forty-six minutes elapsed between the time of the patient’s cardiopulmonary arrest when the first temperature of 38.3°C was hand-collected and the time the next temperature reading of 24.0°C was charted during which time the patient had cooled 14.3°C! The use of a prototype single channel auto-logging thermometer proved critical for acquiring data documenting this patient’s response to a novel method of external cooling.
Despite generation of data acquisition sheets, detailed Standard Operating Procedures (SOPs) and the presence of trained and motivated personnel, complete data (esophageal temperature) were not acquired during critical parts of the patient’s transport. Automated temperature logging and the designation and single-minded training of an individual with sole responsibility for acquiring critical physiological data would seem to be two complementary solutions to this problem. The recent emergence of highly compact, portable, battery operated, “laptop” computers may allow for continuous multi-channel data acquisition of patient temperatures and other parameters providing appropriate transducers, analog to digital conversion hardware, and software become available commercially at affordable rates, or can be generated in-house.
Post-Freeze Examination and Evaluation
As was noted previously this patient appeared to have perfused very well and to have achieved both uniform and high terminal concentration equilibration of cryoprotectant in all of the cephalic tissues by visual examination during and at the conclusion of glycerol perfusion. It has been previously noted that homogenous glycerolization of patients or animals results in the tissues becoming uniformly chalk-white taking on the appearance of cast Plaster of Paris. Inhomogeneous distribution of cryoprotectant or failure of cutaneous cryoprotective perfusion results in a mottled appearance or in the presence of islands of tissue which appear only slightly chalky and retain a bronze or even flesh-colored appearance.
This patient was not examined following freezing due to concerns about inflicting undesirable or even dangerous warming during handling and examination. In the future, it would seem desirable to document the condition of the patient’s skin and the cut surface of the cervical stump with extensive photography to help further evaluate the efficacy of cryoprotective perfusion. Safe documentation of this kind would require the development of an environmental chamber with photographic ports. Additionally, the practice of covering the cervical stump with skin flaps would have to be abandoned so that visualization of the many anatomical structures of interest, in particular the spinal cord (as an indirect indicator of the brain’s response to CPA perfusion), could be carried out.
The author believes this practice should be abandoned for others reasons, principally because it is time consuming, provides no unique protection which could not otherwise be more rapidly and effectively provided (e.g., by a covering dressing), and may interfere with the interface and access of repair devices during future attempts at resuscitation.
Evaluation of Serum Chemistries
Drawing conclusions from the serum chemistry data of this patient must, necessarily, be tentative because this patient represents an n=1 in many ways. As the patient population increases and the associated data base increases with it, more certain and perhaps more meaningful conclusions may be drawn.
A baseline blood sample drawn shortly after the start of CPS (but prior to the administration of any transport medications) is indicative of dehydration and probably of emerging renal failure. The BUN and uric acid are markedly elevated at 47 mg/dl and 8.8 mg/dl, respectively and the serum K+ is 9.7 mEq/l (a potentially lethal level). The serum creatinine is slightly elevated at 1.4 mg/dl (normal = 0.6 to 1.2 mg/dl). The serum total protein (TP), while appearing to be at the lower end of the normal reference range (6.0 to 8.0 g/dl) must be considered in the context of the patient’s profound dehydration and hemoconcentration as indicated by the serum osmolality of 358 mOsm/l. Normovolemic serum TP, consistent with this patient’s poor nutritional status, would be expected to be below 5.0 g/dl. Probable respiratory alkalosis is present with a pH of 7.65.
It is probably fortunate that this patient underwent dehydration in the presence of malnutrition and anemia since this minimized the amount of hemoconcentration and increase in blood viscosity during the agonal period. Patients with normal serum protein concentration and normal or elevated RBC and/or WBC concentration can be expected to experience severe hemoconcentration and associated risks of hypercoagulability and sudden death.
The patient’s expected hyperosmolar state at the time of cardiopulmonary arrest was not as severe as was anticipated. However, in future cases terminal serum osmolality may be much higher. In any event, in the absence of measured osmolality it must be presumed the patient is hyperosmolar if the history or clinical picture is one of dehydration at the time of arrest. This is critically important because the osmolality of the TBW perfusate must be adjusted to accommodate the likelihood of serious hyperosmolality in the patient. Perfusing a patient with a patient with a serum osmolality < or = to 340 mOsm/kg will result in cellular edema, and, in cases of severe hyperosmolality (<400 mOsm/kg) may result in cerebral edema with possible pontine myelolysis [53,54].
In-house survival canine TBW models have shown that there is no adverse neurological or other sequlae associated with the use of perfusates of ~450 mOsm/kg. In fact, the use of hyperosmolar perfusate was essential to good outcome in this model [55]. One clear conclusion to be drawn from this discussion is that there is no downside to the use of hyperosmolar perfusates and considerable potential downside to the use of perfusates of “normal” (~310 mOsm/kg) osmolality.
One of the few advantages to cryopreservation procedures starting concurrent with cardiopulmonary arrest is that production of some serum metabolites and serum lipids ceases due to both ischemia and the rapid induction of hypothermia. Because a baseline blood sample was obtained in this case it possible to use metabolites such as BUN, creatinine and urea as markers for hemodilution by Transport medications and as indicators of the effects of TBW on the vascular, interstitial and even intracellular compartments. Similarly, cholesterol, total protein and serum albumin which are normally confined to the vascular space may be used as indicators of the efficacy of TBW in uniformly displacing blood from the patient and replacing it with an appropriate “intracellular” tissue preservative solution. As can be seen from the sample taken at the end of TBW at 2039 the serum cholesterol (which is completely confined to the vascular compartment even in serious edema) is 0 mg/dl. The BUN and creatinine have declined from 47 mg/dl to 0.4 mg/dl and 1.4 mg/dl to 0.2 mg/dl, respectively.
The blood sample drawn at the start of TBW shows the effect of hemodilution and shift of interstitial ad cellular fluid to the vascular space as a result of the administration of Transport medications most of which are markedly hyperosmolar. Serum osmolality has increased to 417 mOsm/kg, BUN has declined to 34 mg/dl, creatinine to 1.1 mg/dl and cholesterol to 95 mg/dl. Note that the BUN and creatinine have declined by roughly the same amount, but that the cholesterol level has dropped far less, the latter reflecting hemodilution by translocation of interstitial and intracellular fluids. Since the interstitial and intracellular fluids have no mobile cholesterol but do contain BUN and creatinine, the degree of hemodilution indicated by the decrease in the serum cholesterol is probably the more accurate measurement for inferring dynamic increases in vascular volume.
Evaluation of Tissue-Specific Serum Enzymes
However as can be seen from the data, ongoing release of tissue specific enzymes during TBW and subsequent cryoprotectant perfusion was significant. Most enzyme levels had declined to very low or undetectable levels at the conclusion of TBW and rebounded modestly during the cold ischemic period of air transport. However, the levels of these enzymes increased, even in the face of continuous dilution from the addition of CPA concentrate to the recirculating system throughout cryoprotectant perfusion.
There were continuous increases in LDH and alkaline phosphatase which increased markedly as glycerol concentration increased. The relationship between increases in these tissue specific enzymes and increasing glycerol concentration is unclear at this time. This increase may reflect osmotic injury to cells and thus greater membrane permeability from cryoprotective induced dehydration coupled with osmotic extraction of intracellular water into the vascular compartment and, with it, intracellular enzymes.
Of interest is the increase in SGOT and SGPT which were observed during cryoprotective perfusion. Elevation of these transaminases is usually observed as a result of hepatocellular injury. In the case of a neuropatient the liver is not being perfused and thus continuous release of these enzymes might seem paradoxical until it is understood that both SGOT and SGPT are present in significant concentrations in a variety of tissues [56]; in particular in skeletal muscle as the table below shows:
Transaminases are widely distributed in human tissues. The relative concentrations in tissues (serum = 1) are:
|
SGOT
|
SGPT
|
|
| Heart | 7800 | 450 |
| Liver | 7100 | 2850 |
| Skeletal Muscle | 5000 | 300 |
| Kidney | 4500 | 1200 |
| Pancreas | 1400 | 130 |
| Spleen | 700 | 80 |
| Lung | 500 | 45 |
| Erythrocytes | 15 | 7 |
| Serum | 1 | 1 |
As the graphic data shows a similar pattern of continuous release of GGT which is almost exclusively found in the liver was not observed. It thus seems reasonable to assume that the source of the SGOT and SGPT released during cryoprotective perfusion probably originated from the skeletal muscle of the head and upper torso.
Pulsatile Flow
Pulsatile flow was use in this patient because of its reported superiority to continuous flow in cardiopulmomary bypass, particularly in improving microcirculatory perfusion, as indicated by a 30% reduction in perfusion time to cool and rewarm during intraoperative hypothermia as compared to conventional continuous flow perfusion [57]. Pulsatile flow has also been demonstrated to significantly improve cerebral microcirculatory perfusion compared to continuous flow perfusion [58]. An unexpected benefit in this case was the observation that physiologically normal intracranial pulsation of the brain can occur not only in asanguineous deep hypothermia, but that it seems to occur only in cryopatients with little or no cerebral ischemic injury. In previous cases where pulsatile flow has been used cortical pulsations have not been observed the principal difference being that these patients experienced long warm and/or cold ischemic times prior to cryoprotective pulsatile perfusion.
Transport and Cerebroprotective Medications
Since 1985 Alcor has employed a combination of drugs to secure anticoagulation, provide protection against cerebral ischemia-reperfusion injury, prevent return of consciousness and spontaneous cardiac activity during CPS, and maintain pH within physiologically survivable ranges [34]. Only recently has Alcor begun testing this combination of drugs in animal models. Some of the medications such as tromethamine (THAM) (maintain appropriate pH) [59,60] , sodium heparin (anticoagulation) [61], sodium pentobarbital (anesthesia and cerebroprotection) [62,63], metubine iodide(neuromuscular blockage to inhibit shivering and reflexive limb/body movement during cold water immersion) [64], methylprednisolone (reduce ischemic injury) [65,66], chloropromazine (membrane stabilization in hypothermia) [67,68] and 20% mannitol (improve cerebral microcirculation) [69,70] have been validated in canine and feline models as either essential or beneficial to the successful ultraprofound asanguineous hypothermic perfusion in dogs using survival and full neurological recovery as the endpoint [55].
Primary cerebroptrotective drugs have been included in the transport protocol on the basis of positive experimental results in global cerebral ischemia as reported in the peer reviewed literature. Each drug and its putative mechanism(s) of action are briefly discussed and references provided. Ongoing review of the literature suggests that some drugs which appeared promising may be ineffective or even deleterious and it is suggested that these agents be reconsidered for inclusion in cryonics transport protocols.
Deferoxamine is an iron chelator employed to scavenge free iron which is known to liberated in large amounts during global cerebral ischemia presumably from intracellular cytochromes, and possibly hemoglobin. Preliminary studies indicated that deferoxamine was profoundly protective in animal models of global cerebral ischemia [71-73], as well as in the experimental clinical setting following prolonged periods of cardiac arrest before resuscitation [74]. However, more recent research has shown negative results in improving outcome with deferoxamine [75]. Further close scrutiny of clinical trials seems warranted to determine if this drug should remain in the Transport Protocol.
Nimodipine initially demonstrated substantial promise in animal models of both focal and global cerebral ischemia [76-78]. However, recent animal [79,80] and clinical studies employing nimodipine in CPR have shown negative or at best mixed results [81] and markedly decreased MAP and resulting in compromises in both coronary and cerebral perfusion with unfavorable clinical results [82,83]. The potent vasodilatory and hypotensive effects of this drug must be weighed carefully against benefit demonstrated in laboratory settings. Human cryopatients already suffer severely compromised perfusion and refractory hypotension during CPS. The author believes that use of nimodipine should be re-evaluated and that until its hemodynamic effects in the clinical setting are better understood, it should be used cautiously, if at all, in cryopatient Transports.
Vitamin E, and its more stable and water-soluble analog Trolox, have shown great promise in ameliorating post-ischemic encephalopathy and post-ischemic myocardial injury [84-89]. In both clinical and animal models of global cerebral ischemia vitamin E has been shown to be cerebroprotective [90]. Combination of vitamin E with mannitol has shown synergistic protective effects and this combination, known as the Sendai Cocktail [91-93] is widely used in Japan to treat at patients with intraoperative risk of cerebral ischemia. While vitamin E has been shown to be more protective than Trolox in a myocardial model of ischemia-reperfusion injury, its insolubility prevents its clinical application. At this time, only alpha-tocopherol acetate is available in a micellized form. Unfortunately, de-acetylation in the liver (a prolonged process) is required for acetate form of vitamin E to have antioxidant activity. Until such time as an active micellized or liposome encapsulated form of active vitamin E becomes available Trolox would seem to be the best alternative.
The acute response of this patient to CPS was judged excellent as evidenced by the prompt return of agonal gasping, rapid drop in core temperature, adequate EtCO2 and visible improvement of skin color: all signs indicative of good perfusion and ventilation. Subsequent direct visualization of the vigorous pulse in femoral artery on Thumper support during cutdown, as well as the bright red condition of the capillary and venous blood, indicate that this patient continued to perfuse and ventilate well up until the time of TBW. Subsequent analysis of serum/perfusate chemistries as well the renal viability study also bears out the likely cerebral viability of this patient (within the context of contemporary medical criteria) throughout transport.
A review of transport medications calls into question the continued use of both nimodipine and desferoxamine. Continued use of both of these drugs should be carefully evaluated in light of ongoing clinical trials evaluating these drugs in cardiopulmonary cerebral resuscitation.
The author also believes that the rapid transport of this patient via air ambulance following TBW was highly significant in reducing both systemic and cerebral cold ischemic injury. This patient did not exhibit the typical post-air transport rigor mortis and was the first patient observed to have a compliant and pulsating cerebral cortex during pulsatile flow cryoprotective perfusion.
The superior blood washout observed in this patient is undoubtedly due to the larger volumes of washout solution employed in this case (i.e., ~30 liters). An additional contributing factor may have been the use of a lower viscosity dextran-containing washout solution for initial TBW in place of the more viscous ViaSpan. In the future, where possible, a minimum of 30 liters of washout solution should be employed for the average adult (75 kg). Larger volumes of washout solution may be necessary in patients with a greater BSA.
1) Fahy, GM. Cryo-Letters 1984;5:79-90.
2) Swenson RD, et al, Hemodynamics in humans during conventional and experimental methods of cardiopulmonary resuscitation. Circulation. 1988;78(3):630-9.
3) Maier GW, Newton JR Jr., et al, The influence of manual chest compression rate on hemodynamic support during cardiac arrest: high-impulse cardiopulmonary resuscitation. Circulation. 1986;74(6 Pt 2):IV51-9.
4) Maier GW, et al, The physiology of external cardiac massage: high-impulse cardiopulmonary resuscitation. Circulation. 1984;70(1):86-101.
5) Ornato JP, et al, The effect of applied chest compression force on systemic arterial pressure and end-tidal carbon dioxide concentration during CPR in human beings. Ann Emerg Med. 1989;18(7):732-7
6) Unpublished Alcor Life Extension Foundation in-house research.
7) Advanced Cardiac Life Support, American Heart Assoc; American Heart Assoc; 1987.
8) Ornato JP, et al, Rapid change in pulmonary vascular hemodynamics with pulmonary edema during cardiopulmonary resuscitation. Am J Emerg Med. 1985;3(2):137-42.
9) Grundler WG, et al, Observations on colloid osmotic pressure, hematocrit, and plasma osmolality during cardiac arrest. Crit Care Med. 1985;13(11):895-6.
10) McDonald JL. Systolic and mean arterial pressure during manual and mechanical CPR in humans. Annal Emerg Med 1982;11:292-295.
11) Ornato JP. et al. Measurement of ventilation during cardiopulmonary resuscitation. Crit Care Med 1983;11:79-82.
12) Leaf, J. D. (1984), Perfusion: Acute Vascular Obstructions and Cold Agglutinins, Cryonics, #44, (Mar, 1984), p. 20.
13) Smith AU. Studies on golden hamsters during cooling to and rewarming from body temperatures below 0 C. II. Observations during and after resuscitation. Proc Roy Soc B 1956;145:407-426.
14) Harris, SB and Darwin, MG. A possible origin for the burr hole drainage phenomenon. BPI Tech Brief #3 http://www.cryocare.org/index.cgi?subdir=bpi&url=tech3.txt
15) Federowicz, M (aka Darwin, M), et al, Postmortem examination of three cryonic suspension patients Cryonics, September 1984.
16) Zuffa M, et al. The thrombo-embolic disease as a paraneoplastic syndrome. Neoplasma. 1982;29(2):241-4.
17) Lindahl AK, et al. Indices of hypercoagulation in cancer as compared with those in acute inflammation and acute infarction. Haemostasis. 1990;20(5):253-62.
18) Uchiyama T, et al. Studies on the pathogenesis of coagulopathy in patients with arterial thromboembolism and malignancy. Thromb Res. 1990 Sep 15;59(6):955-65.
19) Francis JL. Haemostasis and cancer. Med Lab Sci. 1989;46(4):331-46.
20) Matsubara O, et al. Pulmonary thromboembolism: frequency, site, and vascular change in 103 autopsy cases. Am J Cardiovasc Pathol. 1989;2(4):321-8.
21) Cade, JF. High risk of the critically ill for venous thromboembolism. Crit Care Med. 1982;10(7):448-50.
22) Alfaro, MJ. Prophylaxis of thromboembolic disease and platelet-related changes following total hip replacement: a comparative study of aspirin and heparin-dihydroergotamine. Thromb Haemost. 1986;56(1):53-6.
23) Donovan, C, et al. A dream in his pocket: the cryonic suspension of Eugene Donovan. Cryonics. 1990;10(7):10
24) Weisberg HF. “Unraveling the Laboratory Model of a Syndrome: The Osmolality Model,” Clinician and Chemist. The Relationship of the Laboratory to the Physician, Young DS, Hicks J, Nipper H, et al, eds, Washington, DC: American Association of Clinical Chemistry, 1979, 200-43.
25) Water deprivation test in the dog: maximal normal values. J Am Vet Med Assoc. 1979;174(5):479-83.
26) Leech S and Penney MD. Correlation of Specific Gravity and Osmolality of Urine in Neonates and Adults. Arch Dis Child, 1987, 62(7):671-3.
27) Aufderheide TP, Lurie KG. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32(9 Suppl):S345-51.
28) Bednarczyk EM, et al. Hyperventilation-induced reduction in cerebral blood flow: assessment by positron emission tomography. DICP. 1990;24(5):456-60.
29) Nozari, A, et al. Mild hypothermia during prolonged cardiopulmonary cerebral resuscitation increases conscious survival in dogs. Crit Care Med. 2004;32(10):2110-6.
30) Leonov, Y, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10(1):57-70.
31) Walpoth, BL, et al. Accidental deep hypothermia with cardiopulmonary arrest: extracorporeal blood rewarming in 11 patients. Eur J Cardiothorac Surg. 1990;4(7):390-3.
32) Piepgras, A, et al. Rapid active internal core cooling for induction of moderate hypothermia in head injury by use of an extracorporeal heat exchanger. Neurosurgery. 1998;42(2):311-7; discussion 317-8.
33) Leaf, JD, Case report: Two consecutive suspensions, a comparative study in experimental human suspended animation. Cryonics. 1985:6(11) ;13-38.
34) Darwin, M. Alcor member placed in suspension. Cryonics. 1985:6(4);10-19.
35) Darwin, M. The journey begins: Alcor member enters biostasis. Cryonics. 1987:8(8);14-22.
36) Bridge, S. The cryonic suspension of Alice Black. Cryonics. 1988:9(11);15-25.
37) Edwards ND, et al. Survival in adults after cardiac arrest due to drowning. Intensive Care Med. 1990;16(5):336-7.
38) Husby P, et al. Accidental hypothermia with cardiac arrest: complete recovery after prolonged resuscitation and rewarming by extracorporeal circulation. Intensive Care Med. 1990;16(1):69-72.
39) Sekar TS, et al. Survival after prolonged submersion in cold water without neurologic sequelae. Report of two cases. Arch Intern Med. 1980;140(6):775-9.
40) Martin TG. Neardrowning and cold water immersion. Ann Emerg Med. 1984 Apr;13(4):263-73. Review.
41) Alexander, L. The Treatment of Shock from Prolonged Exposure to Cold, Especially in Water, Combined Intelligence Objectives Subcommittee, Item No. 24, File No. XXVI-37, pp 1-228, July, 1945.
42) Mitscherlich, A, Mielke, F. Doctors of Infamy: The Story of Nazi Medical Crimes. New York, Henry Schuman;1949.
43) Giesbrecht,G. Inhibition of shivering increases core temperature afterdrop and attenuates rewarming in hypothermic humans. Appl Physiol 83: 1630-1634, 1997
44) Vogelaere P. Cardiac output variations in supine resting subjects during head-out cold water immersion. Int J Biometeorol. 1995;39(1):40-5.
45) Bristow, G. K. Clinical aspects of accidental hypothermia. In: Living in the Cold. Physiological and Biochemical Adaptations, edited by C. Heller, X. J. Musacchia, and L. Wang. New York: Elsevier, 1985, p. 513-522.
46) Hayward JS, et al. Physiological responses and survival time prediction for humans in ice-water. Aviat Space Environ Med. 1984;55(3):206-11
47) Griepp RB, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg. 1975;70(6):1051-63.
48) Hayward JS, et al. Thermal and cardiovascular changes during three methods of resuscitation from mild hypothermia. Resuscitation. 1984;11(1-2):21-33
49) Toner MM, et al. Effects of body mass and morphology on thermal responses in water. J Appl Physiol. 1986;60(2):521-5.
50) Jackson, A.S. & Pollock, ML. Generalized equations for predicting body density of men. British J of Nutrition, 1978;40:497-504.
51) Yuhasz, M.S. Physical Fitness Manual, London Ontario, University of Western Ontario, (1974).
52) Sloan AW. Estimation of body fat in young men. J Appl. Physiol. 1967;23:311-315.
53) Go, GM, et al. A case of central pontine myelinolysis and extrapontine myelinolysis during rapid correction of hypernatremia. Rinsho Shinkeigaku. 1994;34(11):1130-5. Review.
54) Kono, S, et al. A case report of central pontine myelinolysis associated with serum hyperosmolality after open heart surgery. Kyobu Geka. 1993;46(2):150-4.
55) Leaf, JD, et al. A mannitol-based perfusate for reversible 5-hour asanguineous ultraprofound hypothermia in canines. Cryovita Laboratories, Inc. and Alcor Life Extension Foundation, unpublished paper 1987.
56) Jiang, ZL, et al. Muscle damage induced by experimental hypoglycemia. Metabolism. 1998;47(12):1472-6.
57) Williams GD, et al. Pulsatile perfusion versus conventional high-flow nonpulsatile perfusion for rapid core cooling and rewarming of infants for circulatory arrest in cardiac operation. J Thorac Cardiovasc Surg. 1979 Nov;78(5):667-77
58) Taylor, KM, et al. Comparative studies of pulsatile and non-pulsatile flow during cardiopulmonary bypass, Journal of Thoracic and Cardiovascular Surgery. 1978;75: 569-73.
59) Bacalzo LV, Jr. and Wolfson SK, Jr. Preferential cerebral hypothermia. Tromethamine and dextran 40 and tolerance to circulatory arrest of 90 to 120 minutes. Arch Surg. 1971;103(3):393-7.
60) Nahas, GG, et al. Guidelines for the treatment of acidaemia with THAM. Drugs. 1998 Feb;55(2):191-224. Review. Erratum in: Drugs 1998;55(4):517.
61) Klebanoff, G, et al. Extracorporal circulation in hypothermia as used for total-body washout in stage IV hepatic coma. Ann Thorac Surg. 1973;16(1):44-51.
62 Barbiturate therapy in cerebral ischaemia. Anaesthesia. 1980;35(3):235-45. Review.
63) Shiu, GK. and Nemoto, EM. Barbiturate attenuation of brain free fatty acid liberation during global ischemia. J Neurochem. 1981;37(6):1448-56.
64) Rodriguez, JL, et al. Physiologic requirements during rewarming: suppression of the shivering response. Crit Care Med. 1983;11(7):490-7.
65) Chatterjee, SN. Pharmacologic agents of potential value in protecting kidneys from ischemic damage. Transplant Proc. 1977;9(3):1579-82. Review.
66) Pavlock, GS. Lysosomal enzyme release in hypothermically perfused dog kidneys. Cryobiology. 1984;21(5):521-8.
67) Tokunaga, Y, et al. Improved survival of kidneys preserved for seven days with a phospholipase inhibitor. Transplant Proc. 1991;23(1 Pt 1):691-2.
68) Tokunaga, Y, et al. Improved rat liver preservation using chlorpromazine in a new sodium lactobionate sucrose solution. Transplant Proc. 1991;23(1 Pt 1):660-1.
69) Shirane R, Weinstein PR. Effect of mannitol on local cerebral blood flow after temporary complete cerebral ischemia in rats. J Neurosurg. 1992;76(3):486-92.
70) Andrews, RJ. et al. Effects of mannitol on cerebral blood flow, blood pressure, blood viscosity, hematocrit, sodium, and potassium. Surg Neurol. 1993;39(3):218-22.
71) Horesh, PA. Iron depletion or chelation reduces ischemia/reperfusion-induced edema in gerbil brains. J Pediatr Surg. 1990;25(2):224-7; discussion 227-8.
72) Kumar K, et al. A quantitative morphological assessment of the effect of lidoflazine and deferoxamine therapy on global brain ischaemia. Neurol Res. 1988;10(3):136-40.
73) Navani, NR, et al. Post resuscitation iron delocalization and malondialdehyde production in the brain following prolonged cardiac arrest. J Free Radic Biol Med. 1985;1(2):111-6.
74) Babbs, CE. Role of iron ions in the genesis of reperfusion injury following successful cardiopulmonary resuscitation: preliminary data and a biochemical hypothesis. Ann Emerg Med. 1985 Aug;14(8):777-83. Review.
75) Fleisher, JL, et al. Failure of deferoxamine, an iron chelator, to improve neurologic outcome following complete cerebral ischemia in dogs. Stroke. 1987 Jan-Feb;18(1):124-7.
76) Larawicz, JW, et al. Diverse mechanisms of neuronal protection by nimodipine in experimental rabbit brain ischemia. Stroke. 1990 Dec;21(12 Suppl):IV108-10.
77) Nuglish, J, et al. Protective effect of nimodipine against ischemic neuronal damage in rat hippocampus without changing postischemic cerebral blood flow. J Cereb Blood Flow Metab. 1990 Sep;10(5):654-9.
78) Sakabe, T, et al. Protective effect of nimodipine against ischemic neuronal damage in rat hippocampus without changing postischemic cerebral blood flow. J Cereb Blood Flow Metab. 1990 Sep;10(5):654-9.
79) Tateishi, A, et al. Failure of nimodipine to improve neurologic outcome after eighteen minutes of cardiac arrest in the cat. Resuscitation. 1991 Apr;21(2-3):191-206.
80) Calle, PA, et al. Nimodipine has no beneficial effect on neurological outcome in a cardiopulmonary arrest model in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jun;341(6):586-91.
81) Roine, R.O, et al. Nimodipine after resuscitation from out-of-hospital ventricular fibrillation. A placebo-controlled, double-blind, randomized trial. JAMA. 1990 Dec 26;264(24):3171-7.
82) Gueugniaud PY, et al. Effects of nimodipine on cerebral blood flow and cerebrospinal fluid pressure after cardiac arrest: correlation with neurologic outcome. Anesth Analg. 1989 Apr;68(4):436-43.
83) Forsman, M, et al. Effects of nimodipine on cerebral blood flow and cerebrospinal fluid pressure after cardiac arrest: correlation with neurologic outcome. Anesth Analg. 1989 Apr;68(4):436-43.
84) Hall, E. of the Central Nervous System Diseases Research Division of the Upjohn Pharmaceutical Company. (Letter) Free Radical Biology and Medicine (4, 135-6 (1988)).
85) Fujimoto, S, et al. The protective effect of vitamin E on cerebral ischemia. Surg Neurol. 1984 Nov;22(5):449-54.
86) Hara, H, et al. Protective effect of alpha-tocopherol on ischemic neuronal damage in the gerbil hippocampus. Brain Res. 1990 Mar 5;510(2):335-8.
87) Yoshida, S. Brain injury after ischemia and trauma. The role of vitamin E. Ann N Y Acad Sci. 1989;570:219-36.
88) Uneohara, H, et al. The protective effect of mannitol, vitamin E and glucocorticoid on ischaemic brain injury: evaluation by chemiluminescence, energy metabolism and water content. Neurol Res. 1988 Jun;10(2):73-80.
89) Mickle, DA, et al. Myocardial salvage with trolox and ascorbic acid for an acute evolving infarction. Ann Thorac Surg. 1989 Apr;47(4):553-7.
90) Massey, KD and Burton KP, Myocardial salvage with trolox and ascorbic acid for an acute evolving infarction. Ann Thorac Surg. 1989 Apr;47(4):553-7.
91) Watanabe, T, et al. [Aneurysm of the extracranial internal carotid artery treated by neck resection and end-to-end anastomosis under the administration of brain protective substances] No Shinkei Geka. 1987 Aug;15(8):893-9.
92) Suzuki, J, et al. Protective effect of phenytoin and its enhanced action by combined administration with mannitol and vitamin E in cerebral ischaemia. Acta Neurochir (Wien). 1987;88(1-2):56-64.
93) Takahasi, A, et al. [Surgical treatment of AVMs occluding these feeders during removal–utilizing the intraoperative balloon catheter and brain protective substances (“Sendai cocktail”)] No Shinkei Geka. 1986 Feb;14(2):179-87.
ADDENDUM
Laboratory evaluations of samples taken during cryoprotective perfusion

A-1049 SGOT (Perfusion)

A-1049 SGPT (Perfusion)

A-1049 Alkaline Phosphatase (Perfusion)

A-1049 Glucose (Perfusion)

A-1049 Calcium (Perfusion)

A-1049 Sodium (Perfusion)

A-1049 Potassium (Perfusion)

A-1049 Chloride (Perfusion)

A-1049 gamma-GT (Perfusion)

A-1049 LDH (Perfusion)

A-1049 BUN (Perfusion)

See also the personal account of this case by Arlene Fried’s daughter:
Her Blue Eyes Will Sparkle


